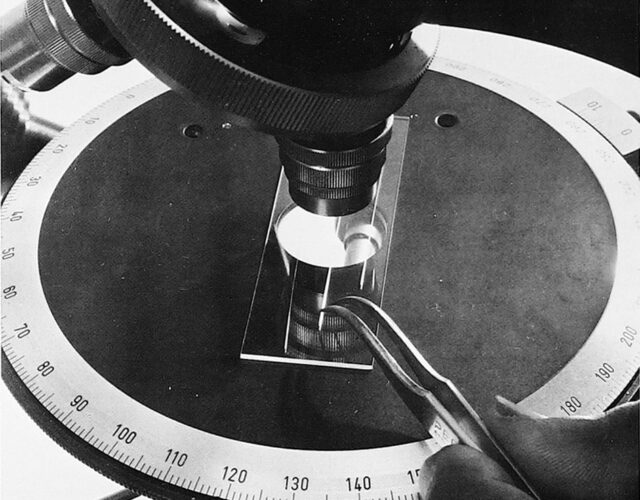
In a backwater Soviet laboratory in the early 1960s, Nikolai Fedyakin toiled away at his research. Fedyakin worked at the technological institute in Kostroma, an old city on the Volga River 200 miles northeast of Moscow. Some would say this bygone hub of the linen industry was charming, others that it was primitive. In many ways this quiet, unadorned corner of the Soviet Union was the perfect setting for the unremarkable research Fedyakin pursued. While other Russian scientists propelled cosmonauts into space, Fedyakin studied water.
Fedyakin was probing an old theory. Back in the 19th century William Thomson, better known today as Lord Kelvin, found that individual water droplets evaporate faster than water in a bowl. Kelvin also noticed water in a glass tube evaporates even more slowly. He surmised that the curvature of the water’s surface affected how quickly it evaporated. To test Kelvin’s theory Fedyakin carefully placed drops of purified water in containers of different shapes. In one experiment he condensed water vapor in a glass tube the diameter of a human hair, sealed it, and stored it upright. When he examined the contents of the tube a few weeks later, he saw something strange. Under the microscope the column of liquid was divided into two parts, separated like vinegar and oil.
Why would water split into two parts, Fedyakin wondered, and did these parts behave in the same way? After repeating his experiments several times under clean laboratory conditions, Fedyakin managed to create a sample size smaller than the equivalent of a drop of dew. His observations were limited by the resolution of his microscope, but he could see enough to realize the liquid at the bottom of the glass tube was denser than ordinary water. Fedyakin published his results in a Russian scientific journal in hopes that others would also find his water curious. Only one man did.
Boris Deryagin was the internationally renowned director of the Institute of Physical Chemistry in Moscow. A tall man with a perpetually pained expression, he had already reached the most rarefied level of Soviet science and was now seeking a research problem that could thrust him into the orbit of a Nobel Prize. The strange water found by an unknown chemist in a forgotten part of Russia was possibly just the boost he needed.
Deryagin struck up a collaboration with Fedyakin and then steadily absorbed the research of the little-known scientist into his own work. Deryagin’s team confirmed the substance at the bottom of the glass tube was denser and thicker than ordinary water. They also discovered that compared with ordinary water it froze at a far lower temperature (–40°F) and boiled at a much higher one (near 400°F). Using an optical microscope the researchers could also see this new type of water expanded more than ordinary water when heated and bent light differently. With every new observation Deryagin grew more convinced that this “modified water,” as he called it, was the most thermodynamically stable form of water, meaning any water coming into contact with the modified water would eventually become modified as well. But Deryagin needed more evidence to prove the water’s newness and strangeness. Once he had that proof, he could catch the attention of scientists outside the Soviet Union.

In 1966, after nearly four years of work, Deryagin got his wish after Soviet authorities permitted him to speak at a conference at the University of Nottingham in England. Most of the conference’s talks elicited contentious debate, but when Deryagin finished speaking, only a few polite questions were asked. His talk, dryly titled “Effects of Lyophile Surfaces on the Properties of Boundary Liquid Films,” was too vague to draw any of the interest usually generated by a scientific breakthrough. The attention he desired, the questions he expected, and the crowds he hoped for never came. Only one British scientist expressed interest in his work: Brian Pethica, the director of the Unilever Research Laboratory in Cheshire, England.
Pethica went back to his group’s laboratory and followed Deryagin’s directions for creating modified water. Three years later Pethica and his team confirmed Deryagin’s findings. But they too were unable to determine the fluid’s chemical makeup apart from the hydrogen combined with oxygen found in ordinary water: the amount of the modified water was below what their instruments could detect. However, using the same tools as the Soviets, they verified the liquid expanded more than ordinary water and found it had a thick, gel-like consistency. They made a cautious guess that this new substance was created by silicates leaching from the glass tube. But they also had their doubts about this theory, as Deryagin had created the water using quartz tubes in which leaching was unlikely. Despite their misgivings they gave the odd liquid a new name—anomalous water—and published a paper about it in Nature, a science journal with a strong international reputation, which alerted American scientists to the discovery.
As Americans devoured the details of this possibly stunning breakthrough, Deryagin finally began to see his Nobel dreams materialize. J. D. Bernal, one of Britain’s most celebrated scientists, told Deryagin that “this is the most important physical-chemical discovery of the century.”
Many less-famous scientists doubted the reality of anomalous water, dismissing it in commentary sections of science periodicals. If anomalous water was the most stable form of water, said these doubters, then all water coming into contact with it should turn anomalous. For some the existence of anomalous water seemed impossible. For others there were data enough to ignite the imagination.
Chemicals, like humans, have unique fingerprints, and instruments called spectrometers can identify the elements and molecules from a chemical fingerprint, or spectrum. Yet success hinges on the size of the sample, where bigger is better. In published papers anomalous-water believers lamented there just wasn’t enough of it, certainly not enough to identify its molecular makeup. Scientists measured what they could with the tiny amounts of anomalous water available, largely physical properties, such as boiling point, appearance, thermal expansion, and viscosity. These observations bolstered their conviction that anomalous water was real, but for every believer there were many more skeptics who loudly dismissed the results. The matter would only be settled by a definitive chemical analysis from a spectrometer sensitive enough to determine the fluid’s chemical composition and structure.

That data arrived on June 27, 1969. A paper published in Science, a prestigious American scientific journal, provided the missing evidence for doubting scientists—definitive spectroscopic proof that this water was different. What made the data even more convincing was the person who led the team, Ellis Lippincott of the University of Maryland, a well-known chemist and an expert in spectroscopy who had built one of the two best spectrometers in the country. Working with polymer chemists from the National Bureau of Standards, Robert Stromberg and Warren Grant, Lippincott showed the liquid’s spectrum was “not . . . of any known substance.” When the scientists tried to chemically analyze the liquid, they found trace quantities of silicon and sodium, in amounts too small to be considered significant. Using the data from these spectrometers the researchers also took a stab at explaining what made the liquid unique: the molecules of H2O, they suggested, were arranged in a honeycomb-shaped network, making a polymer of water. They dubbed it polywater.
Scientists—even the most skeptical—took notice. Polywater also caught the attention of the press and public, some of whom were reminded of Kurt Vonnegut’s monstrous ice-nine from the novel Cat’s Cradle, published a few years earlier. Ice-nine froze whatever liquid H2O it touched, from lakes and rivers to blood and sweat. Like an infection, might the real-life weird water also change any water it came into contact with? And what if polywater were flushed down the toilet? Could it transform ordinary water in a treatment plant, water people might then drink? Physicist Frank Donahoe was also alarmed and vehement about polywater’s threat. “I regard the polymer as the most dangerous material on earth,” he wrote in Nature in October 1969. “Treat it as the most deadly virus until its safety is established.”
Once the press picked up the polywater story, Stromberg started receiving letters, lots of them. “People were writing me that I am destroying the world,” he said.
Polywater’s Soviet origins didn’t help matters. By 1969 the CIA was monitoring polywater research by its Cold War rival, and the Wall Street Journal reported the Pentagon was “bankrolling efforts to push U.S. polywater technology ahead of the Soviet Union’s.”
By 1970 everyone had questions about polywater, and a scientific conference in Bethlehem, Pennsylvania, was advertised to have the answers.
In April 1970 the American Chemical Society held a symposium at Lehigh University, in the steel town of Bethlehem. An entire session focused on water, including anomalous water. A news conference was scheduled to follow. Reporters and the 300 attendees all wanted to better understand the nature of polywater. Passions were high among both skeptics and believers: a fight was simmering.
Deryagin, the godfather of polywater, had the honor of giving the opening talk. After his presentation he was pummeled with questions. One audience member asked about evidence of impurities found in polywater by other labs. “I can’t be responsible for the results that are bad and not by us,” Deryagin replied. If the impurities are present in their equipment, he said, they will turn up in the anomalous water, too.
But Lippincott, the adoptive American father of polywater, said impurities were a problem in his lab and he had had difficulty repeating his earlier findings. Stromberg supported Lippincott’s defection. “With the evidence we had, we started out believing that water forms a polymer,” he said. “New evidence casts serious doubt.”
Denis Rousseau, a 29-year-old postdoctoral scientist at Bell Labs in Murray Hill, New Jersey, was one of the more vocal and passionate disbelievers. He told the heavyweights around him that in working with chemists skilled in detecting trace amounts of compounds, they found the “polywater samples show the material to be highly contaminated.” Results showed “high concentrations of sodium, potassium, carbon, oxygen, and chloride” as well as other compounds. Rousseau was confident impurities were at play in polywater: in one experiment in which he aimed a laser at a sample of polywater, the polywater burned and turned dark brown, a sign the sample contained more than just H2O molecules in a new configuration. “I do not believe there is sufficient evidence to justify a polymer of water,” he said. Deryagin remained unswayed.
Scientists from all over the world had traveled to the Lehigh Valley for an answer, but they left frustrated. The status quo returned as polywater’s supporters got back to the business of making a sample large enough to test, while its skeptics continued to denounce the whole thing as drivel.

Rousseau was determined to prove the nonexistence of polywater, and to make his point he went to the gym, where he believed he could get to the source of the impurities. After an intense game of handball Rousseau wrung out the perspiration from his T-shirt and put a sample of it into his spectrometer. The machine spat out the chemical spectrum, which matched that of an earlier sample of polywater. In January 1971 Science published a paper with Rousseau’s findings. In it he wrote that each person, like the Peanuts character Pigpen, is surrounded by a fog containing a fine mist of that person’s essence. This mist, or aerosol, when it landed on the inside of a glass tube containing a microscopic amount of water, created the fluid with the odd behavior. Polywater turned out to be 1% inspiration and 99% perspiration. Put another way, polywater was merely dirty water.
Finally polywater’s adherents lost their faith. Scientists got back to work left undone while polywater had consumed them along with millions of research dollars. It was all water under the bridge now. Two years later even the stubborn Deryagin conceded. “These experiments do not support the hypothesis of anomalous or polymeric water,” he said.