Arsenic is a byproduct of Earth bubbling from the inside out. Before there was life, and therefore before the possibility of toxicity, arsenic spewed from holes and crevasses in Earth’s crust and bonded with sulfur, iron, and other metals.
Arsenic was an abundant and bioavailable nutrient for organisms that craved metal ions during the anaerobic stages of evolution. It offered biogeochemical pathways for organisms to survive in these environments without oxygen. Some of the earliest microbes on Earth evolved with arsenic released from volcanoes, plate tectonics, and deep-sea vents. They breathed the stuff. It’s perhaps no coincidence, then, that arsenic sits in group 15 of the periodic table as a metalloid, dualistic in nature: metal and nonmetal, life giver and taker.
Oxidized arsenic became toxic to most living things with the arrival of oxygen-breathing organisms around two billion years ago. During the Great Oxidation Event, cyanobacteria began to fill Earth’s atmosphere with oxygen. Life had to develop strategies to combat toxic elements, including arsenic (III), which oxidized into more bioavailable forms, such as arsenate (V). Given the “broad-scale sensitization of microbial life” to arsenate, “protective mechanisms” against the metalloid spread throughout the tree of life. Its toxicity now connects life through pathways of resistance.

The cells of cyanobacteria, fungi, and plants share many things in common; one of them is an aversion to arsenic. Humans, like other animals, are also biologically wired to reject arsenic. However, our societies began to accept arsenic as an essential element for modern world-building during the Industrial Revolution. How these industrial societies transformed this geological byproduct into a source of economic and political power had a lot to do with who was exposed to the benefits of arsenic and who was exposed to its harm. In the Americas, unnatural arsenic exposure increased under settler colonialism and slavery.
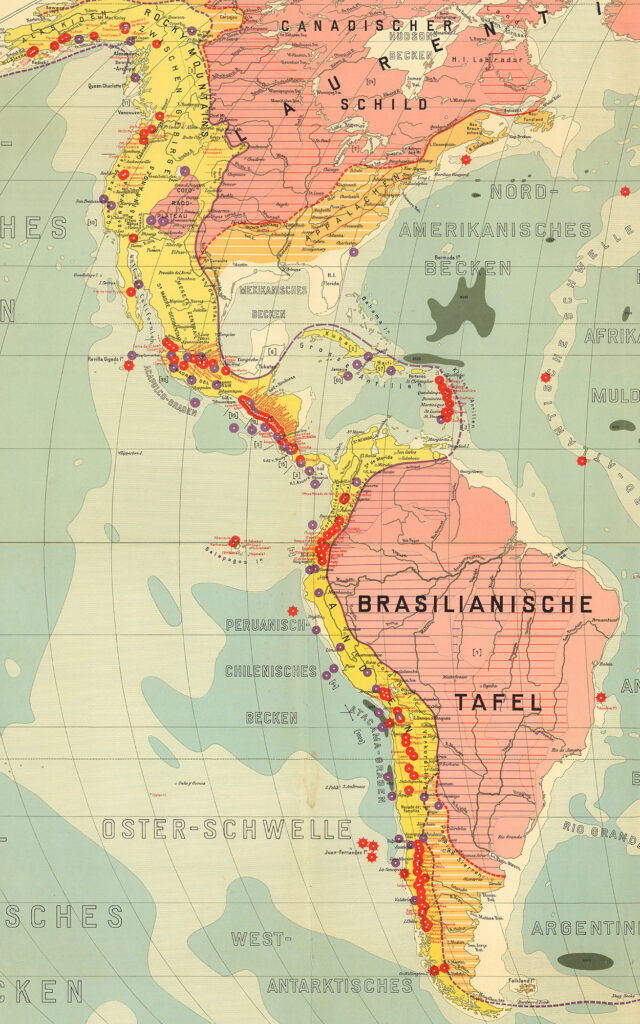
Arsenic contamination follows natural and unnatural pathways in the region. Geographically, arsenic deposits concentrated along the same geological processes that created the Pacific coastal rim of the Americas, from Alaska to Argentina. Millions of years of magmatic activity from the Ring of Fire volcanoes formed the concentration of arsenic deposits in Latin America, western Canada, and the U.S. West.
Untold amounts of arsenic from these deposits have slowly seeped and eroded into river basins, mineral ores, and alluvial aquifers. While most living things on Earth evolved to resist trace amounts of arsenic, life in this part of the world has encountered even higher concentrations. Did the Ring of Fire and its countless arsenic deposits inform life and death in modern Latin America? Mummified bodies from Chile reveal that people have suffered chronic arsenic poisoning for at least 7,000 years.
When hundreds of arsenic scientists and scholars met in Mexico City in 2006 to discuss 100 years of arsenic contamination in Latin America, they acknowledged naturally occurring arsenic as the principal culprit. They noted how colonial mining for silver and gold created new forms of contamination and exposure. However, they agreed that the historical and persistent use of arsenic pesticides was the region’s second primary source of arsenic contamination.
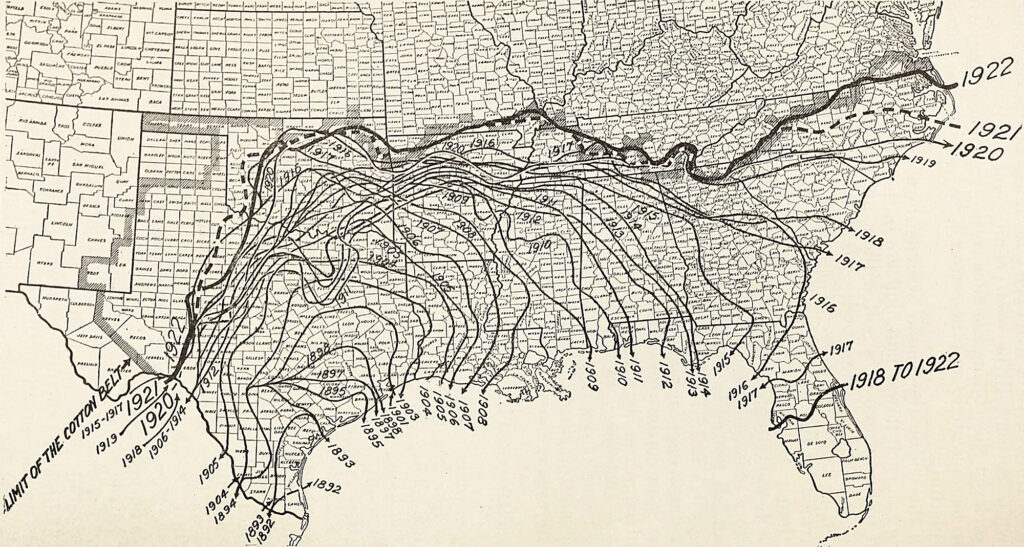
In 500 years, the colonial unearthing of arsenic has continued to put selective pressure on certain organisms in the Americas. The realization that arsenic killed many of the same pests that monoculture attracted to plantations transformed large-scale agriculture and the global arsenic cycle during the export boom in the Americas that lasted from the 1870s to 1930s. Across the hemisphere, planters and politicians looked to chemistry to maintain agricultural production and white supremacy during the gradual abolition of slavery.