Albert Einstein once remarked that “a person who has not made his great contribution to science before the age of thirty will never do so.” J. J. Thomson represents a glaring contradiction to this sentiment. After receiving the Nobel Prize in physics days before his 50th birthday in 1906, Thomson embarked on a new line of research, arguably invented mass spectrometry, and published the field’s foundational text, Rays of Positive Electricity and Their Application to Chemical Analyses, exactly 100 years ago this year.
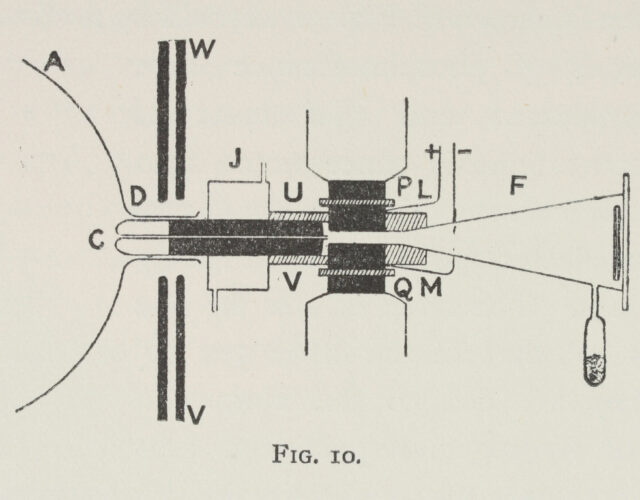
Thomson’s starting point—the “rays of positive electricity’’ from his title—was in a sense the opposite of the electrons (then known as cathode rays) he had won fame for discovering. Originally called Kanalstrahlen, Thomson’s rays had first been described by Eugen Goldstein in 1886. They were produced by drilling channels (Kanäle, or canals) in the cathode of a cathode-ray tube (a sealed glass tube with almost all its air removed and with a cathode and anode added). As the flow of electrons left the cathode, unknown particles flowed in the opposite direction, passing through the holes. At first the rays were enigmatic, since unlike electrons they were not affected by a magnetic field. But researchers soon found that a stronger magnetic field deflected the rays, implying that they were charged particles like electrons but immensely more massive.
Little was known about the nature of these particles until Thomson began a series of improvements to the apparatus. First, he collimated the rays, lining them up by extending the hole in the cathode into a long, narrow tube. Then he developed various techniques to record the faint impression the rays made when they hit the wall of the tube after being deflected by powerful magnetic and electric fields. Ultimately, placing a photographic plate inside the vacuum tube itself proved most effective. Finally, when Thomson reduced the minuscule air pressure inside the tube even further, spots produced by the canal rays suddenly separated and resolved themselves into a series of parabolic smears.
Thomson knew that electric and magnetic fields applied to the rays would cause particles with the same charge-to-mass ratio to deflect as a family, smearing into a unique parabola on the screen. Particles with any other charge-to-mass ratio would make up a distinct family that would form its own parabola. Thomson’s improved apparatus now allowed him to identify the canal rays as positively charged ions with a distinct atomic or molecular mass. He could take the ions of whatever gases were in the tube and spread them across the screen by mass, making a mass spectrum.
Thomson immediately saw an application to chemical analysis. He wrote, “I feel sure that there are many problems in Chemistry which could be solved with far greater ease by this than by any other method. The method is surprisingly sensitive—more so even than that of Spectrum Analysis, [and] requires an infinitesimal amount of material.” The method excelled, for instance, in revealing trace constituents of a sample.
Much of the credit for improving the original apparatus must go to Thomson’s assistant, the talented experimenter Francis William Aston, who joined Thomson’s lab in 1910. Thomson credits Aston early in the book with creating a clever means of photographing the mass spectra but hardly mentions him after that. Ironically, Rays of Positive Electricity does contain the seeds of Aston’s later claim to fame. The last plate of the book includes a photograph of a mass spectrum with an unidentifiable feature corresponding to atomic mass 22 just under the strong line corresponding to neon, which has an atomic mass of 20. “It must, I think, be a new element,” Thomson writes. Aston reappears as Thomson recounts a series of painstaking experiments to separate this new element from the neon so close by. Alas, unable to separate the two because they are so close in weight and physical properties, Thomson voices a suspicion that “the two gases, although of different atomic weights, may be indistinguishable in their chemical and spectroscopic properties.” Aston received his own Nobel Prize in 1922 for the discovery of isotopes, the first of which was neon-22.
Editor’s note: Francis William Aston received his 1922 Nobel Prize for the discovery of non-radioactive isotopes.