Paul Berg didn’t want to answer the question.
Did he regret voicing concern about the safety of his own work?
Did he wonder whether all that had gone awry since he spoke out—the pause in research, the threats of regulation, the public outcry and political grandstanding—could have been avoided?
After unwittingly placing himself at the center of a national controversy over recombinant DNA, the newfound technique that could rearrange the very building blocks of life, had he made the right decision?
“I’ve tried to beg off answering such hypothetical questions,” Berg told journalist Jim Lehrer during a PBS interview in March 1977. “In that time and place it was the right thing to do. I don’t know what I would answer if I had to do it over again.”
Berg was part of a small group of microbiologists and biochemists who a few years earlier had learned how to join DNA from different life-forms and then warned the scientific community of the potential biohazards their methods might spawn. In 1974, at Berg’s urging, scientists voluntarily halted their research—suspending work that pressed on the boundaries of possibility for fear of what lay on the other side.
It was an unprecedented step informed by the recent past. The atomic bomb and the napalm and Agent Orange of the Vietnam War were fresh in the cultural memory. As journalist Janet Weinberg observed, microbiologists recognized their age of innocence was ending too. Or, as one of Berg’s students at Stanford, Janet Mertz, admitted, “I didn’t want to be the person who went ahead and created a monster that killed a million people.”
Just when researchers believed they had found a safe path forward, they were stymied again. The public and politicians caught wind of a cabal of scientists hashing out the future of a technique with immense potential for good and ill, and a debate about responsible scientific practice mutated into a national dialogue that was, at times, decidedly unscientific.
“We are in a rotten mess,” declared geneticist James Watson as he watched the affair devolve into black comedy. Legislators wanted to control research. Activists and even some scientists wanted to ban it. Journalists like Lehrer prodded Berg and his colleagues with questions more rooted in ethics than biology. If Berg wasn’t ready to express regret, Watson, ever the firebrand, had no qualms. The moratorium, he said, was “a silly emotional response which we as scientists should all be ashamed of.”
By 1977, Berg was engulfed in the controversy, his life so consumed by the moral and legal questions recombinant DNA raised that he hardly had time to do the actual work. “For me and many of my colleagues, this exercise in science and public policy has become nightmarish and disastrous,” he wrote to a senator involved in the flurry of congressional debate.

Brooklyn-born and short-tempered, Berg was tired of suffering fools. That included, it seemed, Ethan Signer, a smirking MIT biologist who sat beside him and warned PBS viewers that recombinant DNA was an “invitation for abuse,” one that could lead to “genetic engineering of people, or changing the character of people’s genes.”
Berg listened, jaw clenched. He had just turned 50, and the hysteria’s toll showed in the wrinkles creasing his forehead. He worried that in the furor over recombinant DNA, the opportunity to probe the fundamental nature of human genetics would be lost.
So, Lehrer asked, did he have any regrets? A slight, knowing grin crossed Berg’s face. He declined to answer, but his eyes told the story.
Paul Berg was drawn to biology early. As a child, he yearned to understand how living beings worked. If he came across a dead animal, he brought it home to dissect. In junior high, he read books about science while his parents, immigrants from Russia, read Yiddish newspapers. Paul de Kruif’s Microbe Hunters opened him up to the world of storied microbiologists like Pasteur and van Leeuwenhoek. In time, he would join them.
Berg came of age during a thrilling period of discovery. In 1944, Oswald Avery and colleagues learned that genes were composed of DNA, not protein, as had been believed. Their work was the “first text of a new language,” biochemist Erwin Chargaff said. Chargaff later identified the complementary nature of DNA base pairs, a revelation that Watson, Francis Crick, Rosalind Franklin, and Maurice Wilkins built upon to illuminate the double helix structure in 1953.
In a short time, scientists had begun to read the language through which the living world was expressed. The next step was to try writing with it. Berg, who had just earned a PhD in biochemistry from Case Western Reserve, became an early scribe.
He began his career at Washington University in St. Louis, studying with and teaching alongside Arthur Kornberg, who shared a 1959 Nobel Prize for isolating an enzyme critical in the formation of DNA. Berg followed him to Stanford that same year, soon developing a reputation as a self-assured and principled investigator. He was a stubborn sort who embraced a challenge. “There has never been a fight I’d walk away from,” he once said.
In 1967, when Kornberg successfully replicated the DNA of a small virus in his lab, creating what he called a “primitive form of life” from inert ingredients, the potential of genetic engineering began to surface. Genetic engineering might offer a cure for hereditary defects or control cancer-producing genes, scientists believed, and it would surely deliver a better understanding of life itself. “It opens a wide door to new discoveries in fighting disease and building healthier lives for mankind,” President Johnson said. It also opened a door to the unknown.

During a yearlong sabbatical studying animal viruses, Berg began to wonder whether he could use SV40, a cancer-causing monkey virus, to smuggle bacterial genes into a human cell. It would allow scientists to better understand human gene function, he thought. At the same time, it would cross longstanding evolutionary barriers between species, with unpredictable results.
In the summer of 1971, during a course at Cold Spring Harbor Laboratory, Mertz discussed her work in Berg’s lab—specifically, their plan to transfer SV40 into E. coli—with biologist and cancer researcher Robert Pollack. She had pursued their research cautiously, worried, like Signer, that success could be a step toward eugenics and the genetic engineering of humans. Memories of Nazi experiments lingered on her conscience.
Pollack added other concerns to the conversation. Worried about what might happen if foreign, potentially cancer-causing DNA found its way into the human population via engineered E. coli, he urged Mertz and her fellow students to consider what should be done rather than just what could be done. “Surrender a portion of the scientist’s right to follow his nose without regard to consequences,” he told them. He shared his dismay with Berg, who was indignant but nevertheless backed away from introducing SV40 into E. coli.
Berg focused instead on introducing bacterial DNA into SV40, and in a 1972 breakthrough, he and colleagues spliced a segment of a bacterial virus and three genes of E. coli into the DNA of SV40, using enzymes to cut them open and stitch them back together. It was a cumbersome and complicated process, involving purification of DNA and tedious work with a series of fickle enzymes, but it delivered the first man-made DNA hybrid.
If Berg’s method was inaccessible for an average researcher, that changed the following year. Stanford colleague Stanley Cohen and Herb Boyer of the University of California, San Francisco, streamlined the process. They cleaved and linked genes from two different bacteria using a single enzyme and inserted the molecule into E. coli, where it multiplied. The modified technique, Berg said, “made it possible for anybody to do anything.” In Watson’s mind, the recombinant DNA procedure now “needed only a child’s mind to master.”
With the method simplified, safety concerns grew. Questions surfaced in scientific journals about the risk facing lab workers and the public as experimentation expanded. Berg and Pollack, recognizing the potential for harm, organized a conference in January 1973, gathering nearly 100 scientists to discuss the biohazards of biological research.
They met at the Asilomar Conference Center, a wooded retreat and former YWCA summer camp overlooking the Pacific on California’s Monterey Peninsula. There, the researchers targeted the dangerous lab practices, including pipetting by mouth, that might accidentally let their experiments loose. Their work carried risks, they acknowledged, but nothing beyond their control. They didn’t broach the type of fears Mertz harbored or the ethical quandaries that soon arose, restricting the discussion to biohazards and questions of containment. But it was only a matter of time before deeper anxieties emerged.
In the months that followed, Berg’s phone seemed to ring incessantly. On the other end of the line he would find a scientist asking for the requisite materials to perform the recombinant technique, describing “some kind of horror experiment” they planned to pursue. “You’d ask the person whether, in fact, he’d thought about it, and you found that he really hadn’t thought about it at all,” Berg said.
Each day seemed to bring word of yet another boundary pushed. A student in his own lab even went behind his back to collaborate with Boyer and Cohen to clone vertebrate DNA for the first time. They fused the genes of an African frog and E. coli, which replicated once inserted back into the bacterium. Recombinant DNA had crossed the Rubicon.

“That was scary,” says geneticist Matthew Cobb, author of As Gods: A Moral History of the Genetic Age. “Now you could perhaps start to move DNA from one vertebrate to another. That immediately raised not only fears of new plagues, in the cultural tropes of the time, but also substantial ethical issues with the possibility of human genetic engineering.”
Earlier questions about lab safety quickly evolved into unease among scientists and the science press about how recombinant DNA might be deployed, including in biological weapons. As an article in Science noted, if the public began to doubt the responsibility of the scientific community, research could be curtailed, and trouble would surely emerge.
In the face of so much uncertainty, Berg drafted a letter in July 1974 urging scientists around the world to defer potentially troublesome experiments—specifically, those that might produce antibiotic-resistant, toxic, or cancerous organisms. It was published simultaneously in the three leading journals of the day and co-signed by Boyer, Cohen, Watson, and seven others.
“Ignorance led to fear,” says David Baltimore, a signatory of the so-called Berg letter who set aside his own polio studies using the new technique. “We simply didn’t know what was going to hold back recombinant DNA from causing danger.”
At Watson’s suggestion, the letter announced the authors’ intent to organize a second meeting, in early 1975, to discuss the path forward—and, in his mind, limit the delay in research. Around the world, research was officially or unofficially put on ice until that meeting. It was an unprecedented show of allegiance that hasn’t been replicated in the 50 years since.
Scientists had learned a new language. Now, as they returned to Asilomar, they would decide whether they could be trusted to use it.
Berg had taken a step into the unknown with his letter and the moratorium it created. Uncertain how to navigate this new territory, he studied Albert Einstein’s missive to President Roosevelt in August 1939 warning that “a new and important source of energy” could lead to “extremely powerful bombs.” He reviewed the 1967 Outer Space Treaty that prohibited the use of weapons of mass destruction in space. He searched for precedent to guide the scientific community and found it nowhere. The Asilomar Conference on Recombinant DNA, held over four days in late February, would fill that void for decades to come.
The moratorium had divided the scientific community. There were those, such as Science editor Philip Abelson, who worried that the debate would “disturb the public unnecessarily and could lead to harmful restrictions on all scientific research.” Watson, despite having signed the Berg letter, was already lamenting the halt in research. He staunchly opposed the possibility of recombinant DNA regulation and thought himself a “jackass” for having signed in the first place.

Others, like Nobel laureate John Kendrew, were less sure of scientists’ right to proceed. He believed the incalculable risks of recombinant DNA “may lead us to question scientists’ common and generally unspoken assumption that the acquisition of new knowledge is always an absolute good, requiring no justification, no ethical sanction.”
The conference, then, was an opportunity for scientists to overcome their disagreements well enough to set forth guidelines for the safe resumption of research. It was also their chance to show they could regulate themselves and didn’t need the government’s forceful hand. The responsibility for threading that needle—protecting both science from the public and the public from science—fell to the organizing committee, with Berg as its chair.
More than 140 scientists flocked to Pacific Grove for the conference, just as hundreds of thousands of overwintering monarch butterflies were sweeping out of town. The attendees were mostly men and mostly American, but represented 16 countries in all. They filled Asilomar’s stately redwood chapel with a rush of briefcases, corduroy jackets, and suede shoes as they excitedly shared their research.
With the sun bearing down and shades drawn to let the slide projector shine, the attendees parsed the technical details of the organisms used in their work and contemplated ways to stop toxic agents from escaping the lab. The risks, Berg insisted, were difficult to assess but impossible to ignore. So, too, were the rewards. Conversations weaved between the two. Fears of biological warfare and man-made epidemics were balanced by hopes of nitrogen-fixing crops, biosynthetic insulin, and gene therapies fit to cure untreatable maladies.

Watson, petulant in the face of anything that might inhibit science’s march and doubtful consensus could be achieved, spoke for the incautious among them, announcing his desire to see the moratorium lifted, guidelines or no. The sheer number of questions to consider led to a standstill. What was considered safe and what was dangerous? What should be prohibited, and what constituted a tolerable risk? And who would be at fault if someone got hurt? “We can’t even measure the fucking risks,” Watson scoffed at one point. The impasse threatened to overtake the conference.
“Here we are,” one attendee remarked, “sitting in a chapel . . . trying to create some new Commandments—and there’s no goddamn Moses in sight.”
On the final night, a thick fog encroached from the coast, while one by one a small group of lawyers approached the podium to stir the scientists from their stalemate. “They put the fear of God into the audience,” Berg later recalled. The lawyers laid plain the scientists’ personal liability in the event of an accident or lab leak. They also challenged attendees to look up from their microscopes and consider the public interest. Alex Capron, then a law professor at the University of Pennsylvania, said scientists alone weren’t fit to answer the questions raised by their work.
“This group is not competent to judge the risk-benefit ratio of experiments,” he warned. “That is a social decision, as is the judgment on benefit itself.”

But Berg and his fellow organizers were determined to walk away from Asilomar with something to show for their effort. They demanded their peers recognize their moral responsibility, rather than insist on laboratory liberty at the expense of public welfare. Fueled by Chinese takeout, the leaders stayed up late into the night, a full moon overhead, hammering out a covenant that could guide the entire scientific community in its next steps with recombinant DNA.
In the morning, a weary Berg presented the organizers’ formal recommendations for ending the moratorium and resuming experiments. They encouraged “considerable caution” as scientists set forth under four tiers of research arranged by threat, from relatively innocuous (frog genes in a bacterial cell, for example) to hazardous (cancer-causing genes in a human virus). Each level of research should be conducted in a corresponding containment facility with increasingly strict protocols and fail-safes. And the authors urged continuous review of recombinant experiments and containment procedures so the guidelines could be loosened or tightened as needed.
The document may not have settled all the scientists’ disputes, but nearly everyone in attendance agreed to sign off anyway. The organizers were exhausted but euphoric. “We felt that we’d just written the Declaration of Independence,” Berg said.

By the next day, the National Institute of Health’s Recombinant DNA Advisory Committee, formed in 1974 in response to the growing uncertainty, adopted interim rules based on the Asilomar statement and set out to establish something more permanent. Research in federally supported labs could resume. Progress could proceed.
But once the American public read about Asilomar from the small group of journalists who attended the event, the issue of recombinant DNA burst into the national discourse.
“None of us realized what would ensue,” co-organizer Norton Zinder said, “and none of us realized that our lives would never be the same.”
On June 23, 1976, the same day the NIH issued its first official guidelines for recombinant DNA experiments, the circus began.
Harvard University had applied earlier that year for permission to build a containment facility for genetic research. Cambridge Mayor Al Vellucci, a flamboyant, excitable thorn in the university’s side, relished the chance to raise a bit of hell. The Ivy League scientists might create an incurable disease, he suggested in the press, or even some kind of monster. “Is this the answer to Dr. Frankenstein’s dream?”

Cambridge City Council voted to hold hearings on the proposal, and the national media pounced on the story. Reporters crowded City Hall, where one city official was dressed as the American flag. Skeptical residents packed the room to overflow, beckoned by a flyer made by the activist organization Science for the People, which warned that Harvard scientists were planning “the creation of new and possibly dangerous forms of bacteria.”
The NIH sent Maxine Singer, a biochemist and Asilomar organizer, to testify alongside academics from Harvard and MIT. They were there to explain the processes and benefits of recombinant DNA to the city officials, who instead were left with their heads spinning. The arguments for and against genetic research offered little new to standing debates in the scientific and popular press, but the public arena was a distinct shift from the seaside chapel at Asilomar.
“Whether this research takes place here or elsewhere, whether it produces good or evil, all of us stand to be affected by the outcome,” Vellucci reminded the crowd. The hearing carried on until 1 a.m. Two weeks later the council voted in favor of a three-month moratorium on experimentation and the establishment of a review board—ultimately composed of Cambridge residents, including a nun, an engineer, a community activist, and an ethicist—that could proffer further recommendations.
The board met for months, visiting labs at Harvard and MIT to understand the scope of experiments, staging a debate between Nobel winners Baltimore and recombinant DNA opponent George Wald, and diligently listening to more than 100 hours of testimony. Based on the review board’s recommendations, Cambridge became the first city to pass recombinant DNA legislation, effectively codifying the NIH guidelines at the local level. Municipalities across the country followed suit, contemplating their legislative controls. In Ann Arbor, San Diego, and Princeton, restrictions were debated and proposed.
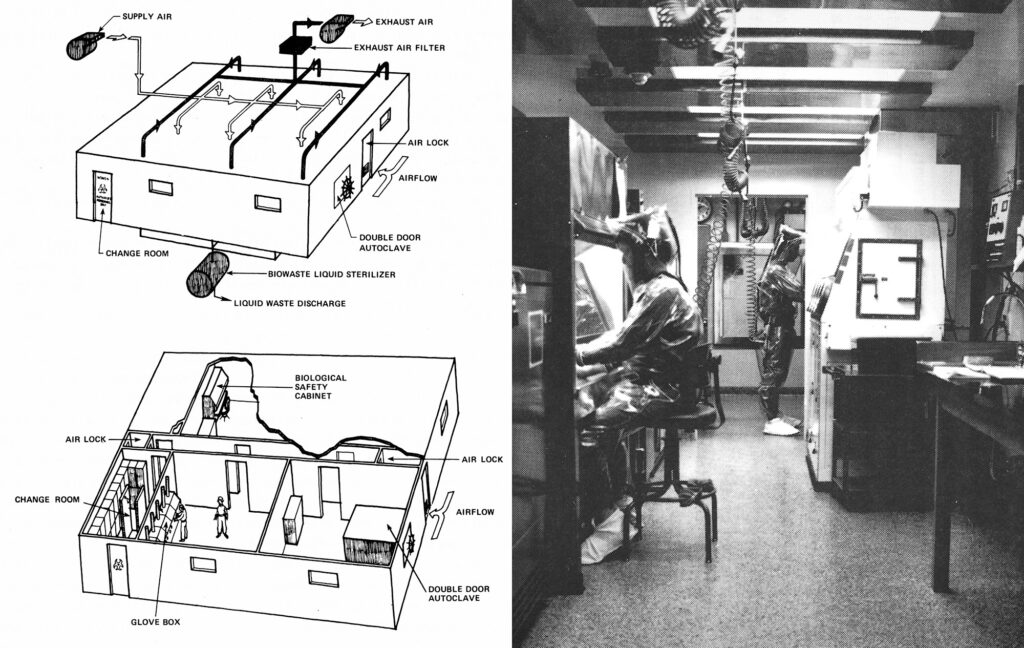
The fate of the field seemed to be spinning out of the scientists’ control. An alarmed Berg raced around the country to offer guidance at city councils, university boards, and public forums, all in a bid to prevent what he called “pre-emptive strikes” that might ground recombinant DNA research before it had truly taken off.
Congress, too, tried its hand. Rep. Richard Ottinger of New York proposed a resolution in early 1977 that declared recombinant DNA “potentially devastating to the health and safety of the American people,” demanding strict adherence to the NIH guidelines with violations punished by fines up to $50,000 and imprisonment. A bill by Edward Kennedy, chair of the Senate’s health subcommittee, proposed an 11-member national commission to regulate and license research facilities, filled in the majority by nonscientists. By 1978 there were 16 separate bills introduced in Congress seeking to regulate research.
Throughout the process, the field’s leaders were put through the ringer. They were called to testify and compelled to speak out in defense of the experiments they had been the first to flag. Berg told Congress that proposals to “govern the content and methods of scientific enquiry” would “stultify the creativity and initiative” that had led to the recombinant technique in the first place and “discourage and disillusion young scientists from entering this field.”

On Lehrer’s PBS program, with the future of genetic engineering still hanging in the balance, Berg sought to win over a wary public. He dismissed the “facetious” arguments against recombinant DNA, such as those leveled by Ethan Signer, the smug MIT professor who compared recombinant DNA to a “big screwdriver” and dismissed its proposed benefits as “pie-in-the-sky.” The work was risky, unpredictable, and dangerous, Signer argued, but the real concern wasn’t safety. Rather, it was more philosophical than that, reflecting an undercurrent that had long rippled beneath the controversy’s surface. “The issue,” Signer said, “is whether we should do this stuff in the first place.”
Watching the conversation around recombinant DNA veer from the science at its center, Berg’s ire grew. He found himself fending off one uninformed politician after another, including a California legislator who had implied Berg supported a bill that would permanently adopt guidelines at least as stringent as those established by the NIH.
“I am unalterably opposed to [the bill’s] intent, astonished by its simplistic and naive assumptions, insulted and dismayed by its provisions, and committed to oppose its enactment,” Berg wrote to the legislator. “The matter [of recombinant DNA] has become politicized, charged with hysteria, and riddled with intrigue, distrust, and cynicism, all of which makes a resolution of the questions that face us more difficult.”

For Berg, those questions remained focused on the safety of experimentation, which was moving forward without him. Cleaning up the mess his own moratorium created had become a second career.
“It was immensely frustrating [for Berg and his colleagues] that they could not engineer the social situation the way that they could engineer life,” says Luis Campos, a science historian at Rice University who is organizing a 50th anniversary summit at Asilomar.
In February 1978, sensing a lull in the storm, Berg saw an opportunity to shift the conversation. He drafted a revision to his 1974 letter, explaining that more than 250 studies had since been conducted involving recombinant DNA molecules with “no indication of harm to humans or the environment.” Scientists on the front lines of the whole affair had seen nothing of the dangers they once feared. Where once he had asked scientists to consider the risks, the past four years had “strikingly altered our assessment,” he wrote.
The updated letter, though, was never published. It wasn’t needed. Kennedy, buffeted by opposition from the scientific community, withdrew support for his own bill. With the worst of the public’s fears failing to emerge, the legislative fervor lost steam. By the end of 1978, the NIH had relaxed its guidelines, loosening restrictions on experiments using E. coli, in particular.
Two years later, in January 1980, the NIH further reduced its restrictions, putting nearly all experiments at the lowest level of concern. By then, the agency was funding more than 1,000 recombinant DNA projects. The following year, the guidelines were made nonmandatory, then relaxed almost entirely. Like that, the whole affair had blown over.

In October 1980, two events revealed just how much had changed in the five years since Asilomar. Genentech, a company co-founded by Boyer in 1976 to capitalize on the recombinant DNA technique he had developed with Cohen, debuted to great fanfare on the New York Stock Exchange, where it immediately doubled in value. The biotech industry had arrived. On the same day, Berg learned he had received the Nobel Prize for his contributions to the science that served as the industry’s foundation.
In his Nobel lecture, Berg said the doubt around recombinant DNA showed “an apprehension about probing the nature of life itself, a questioning of whether certain inquiries at the edge of our knowledge and our ignorance should cease for fear of what we could discover or create.” He urged science to proceed, in characteristic fashion. “I, for one, would not shrink from that challenge,” he said.
For Berg, who died in February 2023, Asilomar became a point of pride as he aged, even if its aftermath left him questioning his decisions in the moment.
“We felt like a bunch of amateurs at the time,” he acknowledged. “We bungled things. But we built some sort of edifice.”
In the conference’s wake, Berg found himself battered for his noble attempt to alert both science and the public to the potential for harm. Perhaps the public’s alarmed response should have been no surprise, but the backlash within the field surely stoked introspection.
The whole affair was “hopelessly overblown,” Watson wrote in 1977, a “tactical mistake” made by paranoid scientists afraid of their own bad dreams. It reminded him of the early-1960s rush to build fallout shelters for nuclear war that never came.

Despite such broadsides, scientists have for 50 years looked to Asilomar when navigating new frontiers—and for good reason, Campos, the science historian, says. Lacking evidence about the risks of recombinant DNA, Berg and his peers made room for disagreement as they sifted through the scientific and moral quandaries that lay before them. Campos sees reflections of the recombinant DNA controversy in our contemporary tangle with artificial intelligence—another technology with the capacity to remake fundamental aspects of our world, for better or worse.
“It’s super powerful and potentially hazardous. So, what do we do?” Campos says. “We have to recover the feeling of that moment, where [the science] was not yet settled, where highly trained folks were landing in different places on this question and changing their positions over time.”
Asilomar, then, offers not just a simple reminder to pause before advancing into the scientific unknown, but a reason to acknowledge the uncertainty that exists at its threshold and consider fully what it means to step forth.
When scientists’ decisions carry implications beyond the lab, Berg believed, questioning the way forward is both a right and a responsibility—and one to be shared with the public as soon as its safety is threatened. The social contract between science and the public—their shared interest in progress—was a force for good, Berg felt, even if Asilomar and its aftermath revealed new strains in that relationship.
In the end “we all came to the same conclusion,” Baltimore says. “Things would have been a lot worse had we not done what we did.”
This story has been updated to clarify that Maxine Singer was not the lone woman to attend the 1975 Asilomar Conference and to remove a characterization of the way lawyers approached the conference’s podium.







