Linus Carl Pauling
Pauling, a Nobel laureate and prolific researcher, made significant contributions to our understanding of chemical bonding and structure.

Best known to the public for championing the use of vitamin C for health purposes and for winning the Nobel Peace Prize, Linus Carl Pauling (1901–1994) was revered by his fellow scientists as a prolific researcher who made significant contributions to our understanding of chemical bonding and chemical structure. His groundbreaking work earned him the 1954 Nobel Prize in Chemistry.
Following the introduction of John Dalton’s atomic theory of matter in the first part of the 19th century, two important questions emerged: what is the nature of of the bond between these atoms that come together to form substances, and how are these atoms geometrically arranged when they bond to form molecules? One hundred years later, following in the footsteps of such scientific giants as August Kekulé, Jacobus Henricus van’t Hoff, and Gilbert Newton Lewis, Pauling made contributions to the fields of chemical bonding and chemical structure that shed significant light on both these questions.
LEARN MORE
Biography is one way of learning about a person. Oral history is another. Spend a few minutes listening to Linus Pauling’s interview archived by the Science History Institute’s Center for Oral History. What do you hear? Has the recording picked up background noises, interesting accents, nervous laughter, or meaningful pauses? What might these tell you about the interview context, who is speaking, or how the speakers feel about the memories being discussed? What do think you can learn about Pauling from his oral history that is different from the content of this biography?
Early Life and Education
Born in Portland, Oregon, Pauling demonstrated an early interest in science by collecting laboratory equipment and conducting chemistry experiments in his childhood home. To earn money in high school, he spent his after-school hours working in the school’s chemistry lab. Despite economic challenges Pauling applied to the Oregon Agricultural College (now Oregon State University), earning his BS in chemical engineering in 1922. He then accepted a graduate appointment at the California Institute of Technology (Caltech), earning his doctorate in physical chemistry and mathematical physics in 1925.
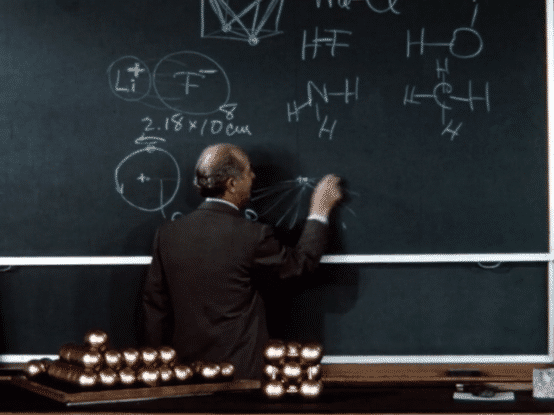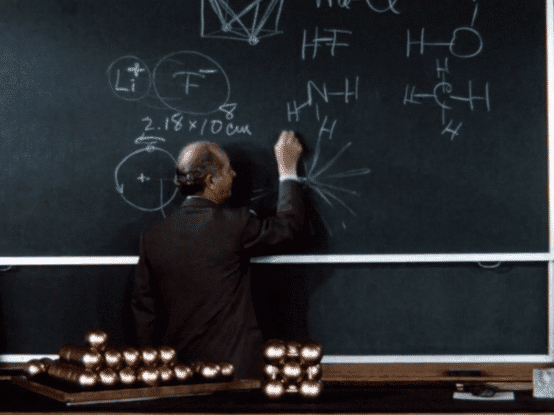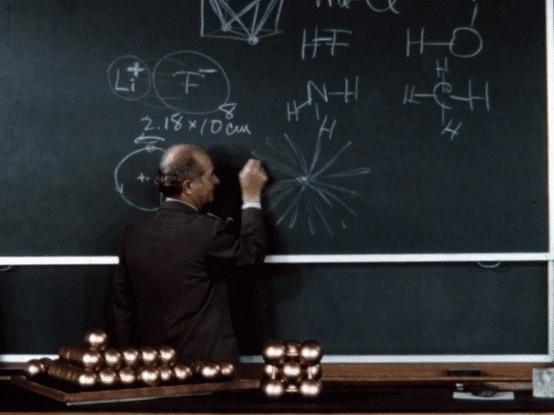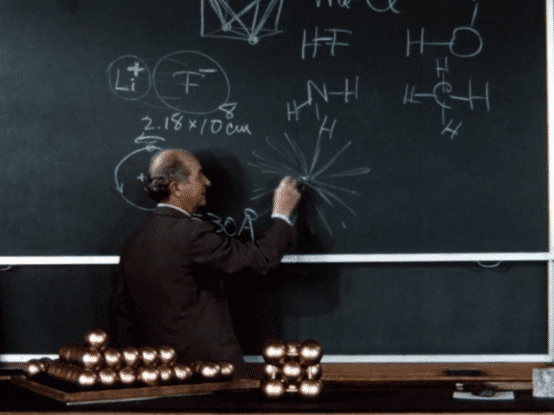
Following a fellowship year in Europe, during which he investigated the implications of the “new physics” of quantum mechanics for chemistry—particularly for chemical structure—Pauling returned to the United States and in 1927 joined the chemistry faculty at Caltech.
Scientific Achievements
During his early years at Caltech, Pauling continued on his trajectory of investigating molecular structures. Using the relatively new technique of X-ray crystallography (also called X-ray diffraction), which he had learned in graduate school, he was able to determine the structures of a number of crystals. Informed by quantum theory, Pauling combined his observations made by X-ray crystallography with complex mathematical calculations to develop generalizations about crystal structures, formulating “Pauling’s rules” for atomic arrangements in crystals with ionic bonding. These rules enabled him and others to more accurately predict and determine crystal structures. Pauling also deciphered the structures of a number of gas molecules using the even newer technique of electron diffraction.
In the mid-1930s Pauling became interested in biological molecules, especially proteins. His investigations of the protein hemoglobin helped him ascertain the molecular cause of sickle-cell anemia and define a new class of disease—molecular disease. In the late 1940s Pauling deduced, from theoretical knowledge and X-ray diffraction data, a fundamental structure of proteins, which he called the alpha helix. For these and other discoveries he is sometimes considered a father of molecular biology.
Pauling conducted pioneering studies in the magnetic properties of atoms and molecules and the relation of electronegativity—the tendency of an atom to attract electrons in a bond—to the types of bonds that atoms form (ionic, covalent, or somewhere in between). He developed the first electronegativity scale to assign values to atoms involved in “intermediate” bonds.
To better explain the nature of covalent bonding, in which electrons are shared between bonded atoms, Pauling formulated the groundbreaking concepts of resonance and hybridization, which in turn provided chemists with a more robust theoretical basis for predicting new compounds and chemical reactions. He later extended the theory of covalent bonds to include metals and intermetallic compounds.
In addition to hundreds of scientific articles Pauling published two seminal works in chemistry. In 1939 his landmark book, The Nature of the Chemical Bond and the Structure of Molecules and Crystals, laid out all of his discoveries to date. And after years of teaching budding young chemists at Caltech, he produced his influential textbook General Chemistry (1947), which changed the way chemistry was taught on a global scale.
Pauling received the Nobel Prize in Chemistry in 1954 “for his research into the nature of the chemical bond and its application to the elucidation of the structure of complex substances.”

Efforts for Health and Peace
In addition to his contributions to our understanding of molecular disease, Pauling also elucidated the roles of antigens and antibodies in the immune system. But he is perhaps best known to the public for championing the use of vitamin C to maintain and restore health (for more on vitamin C, see Albert Szent-Györgyi).
In the 1950s and afterward Pauling campaigned tirelessly—and in the face of significant professional and governmental opposition—to put an end to nuclear-bomb tests in the atmosphere and to the arms race. In 1963, the year that the Nuclear Test Ban Treaty went into effect, Pauling was given the Nobel Peace Prize (although given in 1963, the award was for the year 1962). Pauling was the second person, after Marie Curie, to win two Nobel Prizes.
Pauling stayed at Caltech from 1927 to 1963. Following receipt of the Peace Prize, he spent four years as a research professor at the Center for the Study of Democratic Institutions in Santa Barbara, California. He resumed his scientific research at the University of California, San Diego, in 1967, and from there moved on to Stanford University and finally to the Linus Pauling Institute of Science and Medicine in Palo Alto.
You might also like

DISTILLATIONS MAGAZINE
Linus Pauling’s Vitamin C Crusade
The path to a dubious cure.

SCIENTIFIC BIOGRAPHIES
Albert Szent-Györgyi
Szent-Györgyi and his colleague Walter Haworth were able to synthesize what they called hexuronic acid—today known as ascorbic acid, or vitamin C.

DISTILLATIONS MAGAZINE
The Age of Scurvy
In a time of warring empires and transoceanic voyages, sailors dreaded scurvy more than any other disease.



