Herbert W. Boyer and Stanley N. Cohen
By inventing recombinant-DNA technology, Boyer and Cohen jump-started the biotechnology industry, including Genentech, which creates important applications for a wide range of medical uses.

Recombinant-DNA (rDNA) technology—the way in which genetic material from one organism is artificially introduced into the genome of another organism and then replicated and expressed by that other organism—was invented largely through the work of Herbert W. Boyer, Stanley N. Cohen, and Paul Berg, although many other scientists made important contributions to the new technology as well.
Boyer’s Work with rDNA and Bacteria
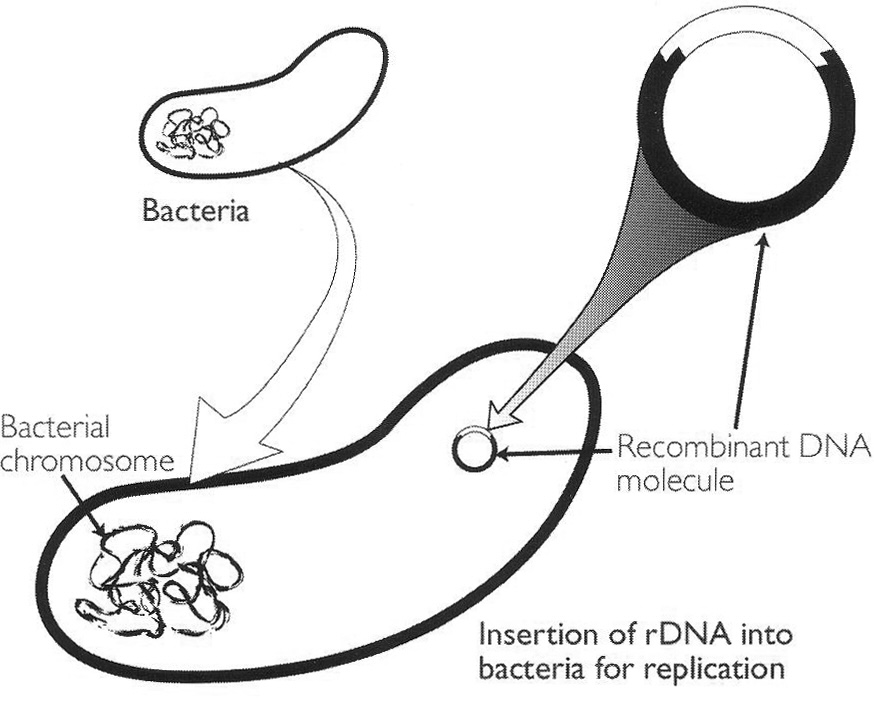
After Paul Berg’s 1971 landmark gene-splicing experiment, the next landmark in the development of modern biotechnology was the insertion of rDNA into bacteria in such a way that the foreign DNA would replicate naturally (see Figure). This step was taken in 1972 by Boyer (b. 1936) at the University of California, San Francisco (UCSF), in collaboration with Cohen (b. 1935) of Stanford University.
Born and raised in western Pennsylvania, Boyer attended St. Vincent’s College in Latrobe, where he enrolled in premedical studies. He was soon captivated by research, however, and chose to major in chemistry and biology. He completed a PhD in biochemistry at the University of Pittsburgh and then went on to a postdoctoral fellowship at Yale University. In 1966 he accepted an appointment at UCSF, which was becoming a center of excellence in the several disciplines that contributed to the emerging field of biotechnology.
In 1972 researchers, including Boyer, realized that the enzyme EcoRI, which had actually been discovered in Boyer’s UCSF lab, cut DNA in such a way that the ends were not blunt but staggered, so that no molecular additions were needed to make one severed piece latch on to another piece possessing complementary cuts. Boyer and a colleague, Robert Helling, began their effort to create rDNA to insert in the bacteria Escherichia coli by trying to use EcoRI to open up the DNA of the bacterial virus lambda. They became frustrated, however, when the enzyme cut the DNA in five places instead of one, as desired.
A Boyer-Cohen Collaboration
November 1972 found both Boyer and Cohen in Hawaii giving papers at a U.S.-Japan joint meeting on plasmids. A plasmid is DNA, found especially in bacteria, that is physically separate from and can replicate independently of the bacterium’s chromosomal DNA. While Boyer was describing his data showing the nature of the DNA ends generated by EcoRI cleavage, Cohen was reporting on a procedure recently discovered in his laboratory that enabled bacteria to take up plasmid DNA and produce offspring that contained self-replicating plasmids identical to the original implant—clones. Over sandwiches late one night at the conference, the two men laid plans for a collaborative project to discover what genes are present on plasmids and how they are arranged.
A native of Perth Amboy, New Jersey, Cohen received his undergraduate education at Rutgers University and then proceeded to the University of Pennsylvania for an MD. After completing his medical education he began a full-time career in medical research and teaching at the Albert Einstein College of Medicine in New York. There he worked on the complex mechanisms that control gene expression in the bacterial virus lambda. In 1968 he accepted an appointment at the Stanford University School of Medicine.
The collaboration between Boyer and Cohen was very close. Plasmids isolated at Stanford were transported to Boyer’s lab in San Francisco for cutting by EcoRI and for analysis of the DNA fragments. These were transported back to Stanford, where they were joined and introduced into E. coli, where they multiplied. Then the brand-new recombinant plasmids were isolated and analyzed in each laboratory.
Interspecies Cloning

The first success of the Boyer-Cohen collaboration occurred in spring 1973 and involved one of Cohen’s plasmids, pSC101. Plasmids were already known to transfer drug resistance among bacteria, and this one could make E. coli resistant to the antibiotic tetracycline. The plasmid pSC101 was cleaved by EcoRI at only one site, leaving intact the plasmid’s ability to replicate. When the linearized pSC101 DNA was mixed with other DNA that had been cleaved by the same enzyme, the complementary ends of fragments from both sources of DNA joined together into new loops. Treatment with another enzyme closed the still-visible nicks in the DNA loops, which were then introduced into calcium chloride–treated bacteria. The bacteria were spread on a culture containing tetracycline, and only the bacteria with the rDNA plasmids survived.
Boyer and Cohen soon moved on to more complicated cloning experiments. They joined tetracycline-resistant plasmids with kanamycin-resistant plasmids—kanamycin being another antibiotic—and inserted them in E. coli. Next they showed that genetic materials could indeed be transferred between species, thereby disproving a long-held myth. They snipped a piece of Staphylococcus plasmid (Staphylococcus is the bacteria responsible for staph infections), spliced it with one of the many E. coli plasmids, and inserted the whole in E. coli. The DNA from Staphylococcus, a different species of bacteria, was successfully propagated in E. coli. An even greater triumph of interspecies cloning was the insertion into E. coli of genes taken from the South African clawed frog.
Birth of the Biotech Industry
Commercial ventures quickly started up with the objective of capitalizing on Boyer and Cohen’s new rDNA technology, despite ongoing controversy over the technology and public fear of cloning. At the forefront was Genentech, founded in 1976 by Boyer and a young venture capitalist, Robert Swanson.
Boyer and Cohen, as well as other scientists involved in cloning experimentation, soon recognized the feasibility of using bacteria into which human genetic information was incorporated to duplicate the body’s natural means of fighting disease and to remedy birth disorders. In fall 1977, even before Genentech had its own facilities, Boyer at UCSF and Keiichi Itakura at the City of Hope Medical Center in Duarte, California, succeeded in expressing a mammalian protein in bacteria—somatostatin. This hormone, produced in the human brain, plays a major role in regulating the growth hormone. Recombinant somatostatin was shown to be virtually identical to the naturally occurring substance.
Recombinant Insulin and Other Projects
In 1978 Boyer and Itakura also constructed a plasmid that coded for human insulin. By then they had many rivals, some of them small start-ups backed by large pharmaceutical companies. In the case of recombinant insulin Eli Lilly and Company signed a joint-venture agreement with Genentech to develop the production process for Humulin. In 1982 Humulin was approved by the FDA, and it became the first biotechnology product to appear on the market.
Whereas Boyer became deeply involved in this commercial work, Cohen remained an academic researcher, focusing on basic questions in genetics and biology. Over the years, however, Cohen has consulted for several biotechnology companies.
Recognition
Boyer received the Biotechnology Heritage Award in 2000 from the Chemical Heritage Foundation, now the Science History Institute, and the Biotechnology Innovation Organization. The award acknowledged Boyer’s distinguished academic career, his development of rDNA technology, his vision as a cofounder of Genentech, and his role in the emergence of biotechnology, which has brought new hope to millions worldwide. Cohen was honored with the 2016 Biotechnology Heritage Award in recognition of his revolutionary work in creating rDNA plasmids, a foundation of all biotechnology. Boyer also received the Society of Chemical Industry’s Perkin Medal in 2007 and the Winthrop-Sears Medal in 2005.



