August Kekulé and Archibald Scott Couper
Kekulé famously “saw” carbon atoms joining in a “giddy dance” in a daydream. Couper invented a symbolic language to represent carbon linkage. Both made significant contributions to the field of structural chemistry.

In 1858 August Kekulé (1829–1896) and Archibald Scott Couper (1831–1892), two young men from different backgrounds, independently recognized that carbon atoms can link directly to one another to form carbon chains. This finding explained the very multiplicity of carbon compounds that had been puzzling chemists.

The discovery by these two scientists depended on Kekulé’s theory, proposed in 1857, that carbon is tetravalent, valence being defined at the time as the combining capacity of atoms of the various elements. Couper, in his paper—and in another paper on salicylic acid that appeared earlier in 1858—indicated valence bonds as straight lines linking the symbols for the elements, which is still the practice in most modern structural diagrams.
Kekulé’s Daydream
Kekulé, a German of Czech descent, was intended by his family to become an architect, but at the University of Giessen he was lured to chemistry by the lectures of Justus von Liebig.
After receiving his doctorate from Giessen, Kekulé served as a research assistant, first in France, then in Switzerland, then in England. In Paris he associated with Charles Gerhardt, and in England with Alexander Williamson, two leading innovators in the effort to understand the constitution of organic compounds.
As he told the story much later, Kekulé envisioned his earliest notion of carbon chains on an omnibus ride in London on the way home from visiting a chemist friend, probably in the summer of 1855. In a daydream he “saw” carbon atoms joining in a “giddy dance.”
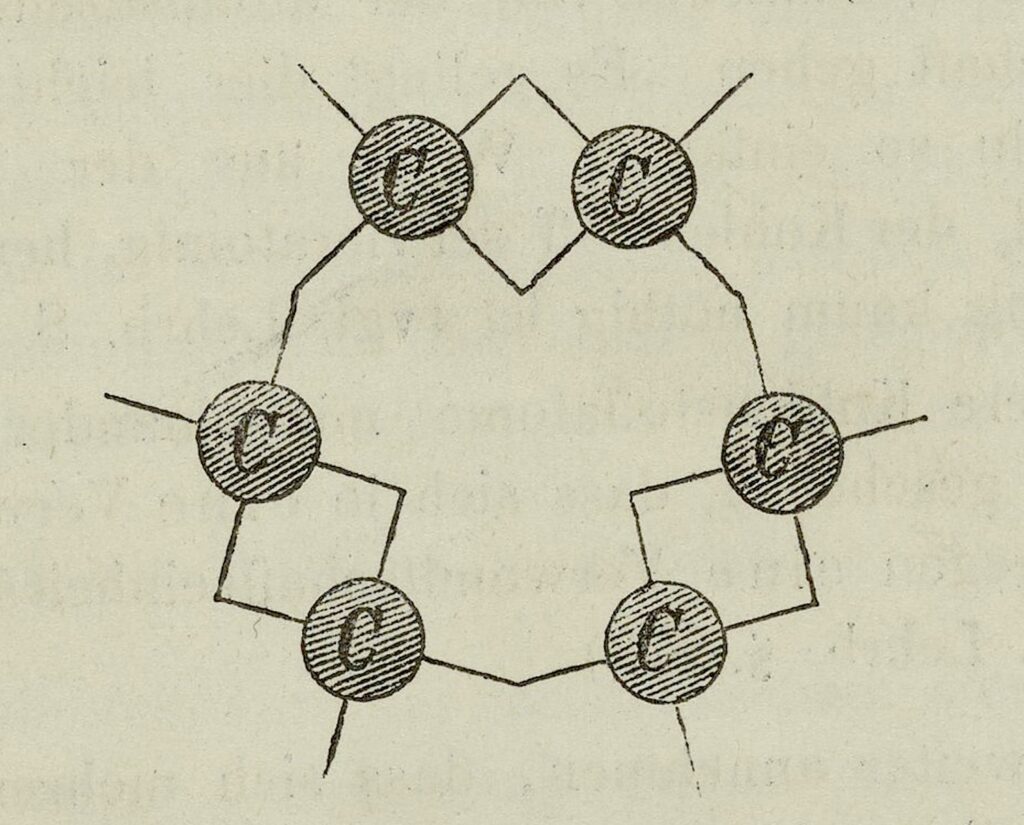
Couper’s Symbolic Representation of Carbon Linkage
Couper, who was a Scot educated in Glasgow, Edinburgh, Berlin, and Paris, came to chemistry from the study of philosophy and classical languages. This background probably helped him make an analogy between letters in words and carbon atoms in molecules, and focus on how carbon atoms combine with other atoms. His invention of an appropriate symbolic language to indicate the order in which the various atoms are joined in molecules may also stem from his philosophical and linguistic training.
Sadly for Couper, his paper describing carbon linkage was read before the French Academy a few weeks after Kekulé’s similar paper was published in Liebig’s Annalen der Chemie und Pharmacie. Couper had entrusted his paper to Charles Adolphe Wurtz, in whose laboratory he worked in Paris, and Wurtz had procrastinated in giving it to an Academy member for presentation.
It is not known how much Couper’s bitterness over his loss of priority and his subsequent fight with Wurtz contributed to his emotional collapse. He soon retreated to his Scottish home and never published another scientific paper for the remaining 30 years of his life.
The Snake with Its Tail in Its Mouth: Benzene
Kekulé returned to Germany from England to begin his career as a university teacher, which took him from the University of Heidelberg, to the University of Ghent in Belgium, and finally to the University of Bonn, where he oversaw the establishment of a chemical institute.
He was a good lecturer, well liked by his many students. As with several other scientists in chemical history, writing a textbook—Lehrbuch der organischen Chemie—proved to be a stimulus for new chemical theories.
Again according to his later reminiscences, one evening in his residence in Ghent (probably in 1862), while he was working on his textbook, he became sleepy and turned his chair to the fire.
Once more he saw dancing strings of carbon atoms, but this time he saw one that, snakelike, took its tail into its mouth, which gave him the idea for the ring form of the benzene molecule. Here then was a structure for the many molecules that would not fit into the existent structural theory.



