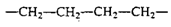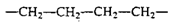Science of Plastics
Definition
Plastics are a group of materials, either synthetic or naturally occurring, that may be shaped when soft and then hardened to retain the given shape. Plastics are polymers. A polymer is a substance made of many repeating units. The word polymer comes from two Greek words: poly, meaning many, and meros, meaning parts or units. A polymer can be thought of as a chain in which each link is the “mer,” or monomer (single unit). The chain is made by joining, or polymerizing, at least 1,000 links together. Polymerization can be demonstrated by making a chain using paper clips or by linking many strips of paper together to form a paper garland.
Examples
Naturally occurring polymers include tar, shellac, tortoiseshell, animal horn, cellulose, amber, and latex from tree sap. Synthetic polymers include polyethylene (used in plastic bags); polystyrene (used to make Styrofoam cups); polypropylene (used for fibers and bottles); polyvinyl chloride (used for food wrap, bottles, and drain pipe); and polytetrafluoroethylene, or Teflon (used for nonstick surfaces). Although many polymers are hydrocarbons that contain only carbon and hydrogen, other polymers may also contain oxygen, chlorine, fluorine, nitrogen, silicon, phosphorus, and sulfur.
Natural polymers, such as cellulose and latex, were first chemically modified in the 19th century to form celluloid and vulcanized rubber. The first totally synthetic polymer, Bakelite, was produced in 1907. The first semisynthetic fiber, rayon, was developed from cellulose in 1911. However, it was not until the global disruption caused by World War II, when natural sources of latex, wool, silk, and other materials became difficult to obtain, that synthetics were mass produced. Synthetic rubber was needed for tires, and nylon was needed as a replacement for silk for parachutes. Today synthetic polymers in the form of plastics are in wide use, and the plastics industry is one of the fastest growing in the United States and around the world. The industry produces approximately 150 kilograms of polymers per person annually in the United States.
Structure
Monomers can be chemically joined together in two ways: addition polymerization or condensation polymerization. Addition polymerization has three basic steps: initiation, propagation, and termination. In this type of polymerization the monomers join by adding on to the end of the last “mer” in the chain, just like making a chain of paper clips. Polyethylene, polystyrene, and acrylic are examples of plastics formed by addition polymerization. These polymers are often thermoplastic in nature: they can be heated and made soft and then hardened when cooled. They are easily processed, reprocessed, or recycled. See the attached tables, Some Addition Polymers and Some Condensation Polymers, for examples of each type.
During condensation polymerization a small molecule is eliminated as the monomers join together. Nylons, some polyesters, and urethanes are examples of condensation polymers. These polymers can be thermoplastic or thermosetting. Although all plastics are in a liquid state at some point in processing and are solid in the finished state, once a thermoset polymer is formed, it cannot be melted and reformed.
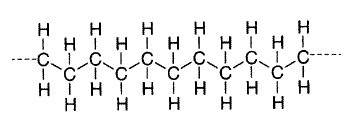
The monomers in a polymer may be arranged in a variety of ways. For example, the monomers may have a linear arrangement like a long chain of paper clips, although the tetrahedral carbon bonds actually give the chain a zigzag configuration. Polyethylene is the simplest example of a linear polymer.
Polyethylene Zigzag Structure
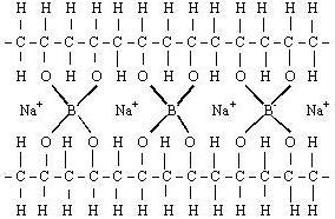
If the monomers not only form straight chains but also form long side chains off the main backbone, the resulting polymer is described as branched and may look like a tree branch or the stems of a bunch of grapes. Another arrangement occurs when the long chains are chemically linked together, forming a mesh-like structure known as a crosslinked configuration. Vulcanized rubber, which is formed by reacting natural rubber (isoprene) with sulfur, is an example of a crosslinked polymer.
The polyvinyl alcohol is cross-linked using borax, Na2B4O7x10H2O (sodium tetraborate).
The polymer molecules can also have different arrangements. If the arrangement has no particular order or form, like the arrangement of spaghetti on a plate, the polymer is said to be amorphous (having no shape). Amorphous polymers are often transparent and, therefore, are used as food wrap, headlights, and contact lenses. These materials also tend to have lower melting points.
If the arrangement is in a distinct pattern, the polymer is said to be crystalline. The higher the degree of crystallinity, the less light passes through. Such materials are either translucent or opaque. This quality depends on the degree of crystallization and the presence of additives. Crystalline polymers have greater strength and tend to have higher melting points.
Characteristics of Polymers
Polymers seem to have a limitless range of characteristics along with properties that allow them to be dyed in an endless array of colors. Their properties can be enhanced by additives. Being able to design or engineer polymers for specific applications makes plastics unique materials. Although each polymer has unique characteristics, most polymers have some general properties:
- They are resistant to chemicals
- They are insulators of heat and electricity
- They are light in mass and have varying degrees of strength
- They can be processed in various ways to produce fibers, sheets, foams, or intricate molded parts
The raw material for manufacturing plastic products is called a resin. Some of the most common resins are polyethylene (PE), polyethylene terephthalate (PET), polypropylene (PP), polyvinyl chloride (PVC), and polystyrene (PS). These resins are often used in packaging.
Some Additional Polymers
|
Polymer name |
Monomer(s) |
Polymer |
Use |
|
Polyethylene
|
CH2=CH2 (ethene) |
-CH2 -CH2– |
Most common polymer. Used in bags, wire insulation, and squeeze bottles
|
| Polypropylene |
CH2=CH ½ CH3 (1-propene)
|
-CH2-CH- ½ CH3 | Fibers, indoor-outdoor carpets, bottles |
|
Polystyrene |
CH2=CH ½
(styrene)
|
-CH2-CH- ½
| Styrofoam, molded objects such as tableware (forks, knives, and spoons), trays, videocassette cases |
|
Polyvinyl chloride (PVC) |
CH2=CH ½ Cl (vinyl chloride)
|
-CH2-CH- ½ Cl | Clear food wrap, bottles, floor covering, synthetic leather, water and drain pipe |
|
Polytetrafluoroethylene (Teflon) |
CF2=CF2 (tetraflouroethene)
| -CF2-CF2– |
Nonstick surfaces, plumbing tape, chemical-resistant containers and films
|
|
Polymethyl methacrylate (Lucite, Plexiglas) |
CO2CH3 ½ CH2=C ½ CH3 (methyl methacrylate)
|
CO2CH3 ½ -CH2-C- ½ CH3
| Glass replacement, paints, and household products |
|
Polyacrylonitrile (Acrilan, Orlon, Creslan) |
CH2=CH ½ CN (acrylonitrile)
|
-CH2-CH- ½ CN |
Fibers used in knit shirts, sweaters, blankets, and carpets
|
|
Polyvinyl acetate (PVA) |
CH2=CH ½ OOCCH3 (vinyl acetate)
|
-CH2-CH- ½ OOCCH3
| Adhesives (Elmer’s glue), paints, textile coatings, and chewing gum |
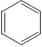
| Natural rubber |
CH3 ½ CH2=C-CH=CH2 (2-methyl-1,3-butadiene)
|
CH3 ½ -CH2-C=CH-CH2– | Rubber bands, gloves, tires, conveyor belts, and household materials |
|
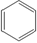
Polychloroprene (neoprene rubber) |
Cl ½ CH2=C-CH=CH2 (2-methyl-1,3-butadiene)
|
Cl ½ -CH2-C=CH-CH2–
| Oil- and gasoline-resistant rubber |
|
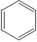
Styrene butadiene rubber (SBR) |
CH2=CH ½
CH2=CH-CH=CH2
|
-CH2-CH-CH2-CH-CH-CH2– ½
| Non-bounce rubber used in tires |
Some Condensation Polymers
|
Polymer name |
Monomers |
Polymer |
Use |
|
Polyamides (nylon)
|
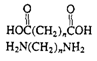
|
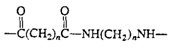
|
Fibers, molded objects |
|
Polyesters (Dacron, Mylar, Fortrel) |
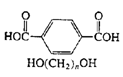
|
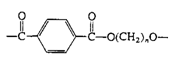 | Linear polyesters, fibers, recording tape |
|
Polyesters (Glyptal resin) |
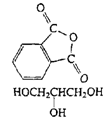
|
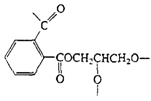 | Cross-linked polyester, paints |
|
Polyesters (Casting resin) |
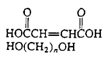
|
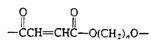 | Cross-linked with styrene and benzoyl peroxide, fiberglass boat resin, casting resin |
|
Phenol-formaldehyde (Bakelite) |
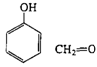
|
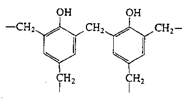 | Mixed with fillers, molded electrical cases, adhesives, laminates, varnishes |
|
Cellulose acetate (cellulose is a polymer of glucose) |
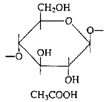
|
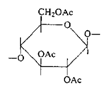
| Photographic film |
|
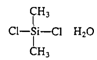
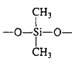
Silicones
|
|
| Water-repellent coatings, temperature-resistant fluids and rubber |
|
Polyurethanes
|
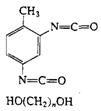
|
| Foams, rigid and flexible, fibers |
Recycling Codes for Plastic Resins
|
Recycling code |
Polymer and structure |
Uses |
 |
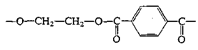
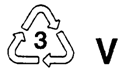
Poly(ethylene terephthlate) (PET) | Bottles for soft drinks and other beverages |
 |
High-density polyethylene | Containers for milk and other beverages, squeeze bottles |
 |
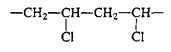
Vinyl/polyvinyl chloride | Bottles for cleaning materials, some shampoo bottles |
 |
Low-density polyethylene May have some branches | Plastic bags, some plastic wraps |
 |
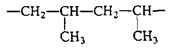
Polypropylene | Heavy-duty microwavable containers |
 |
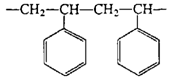
Polystyrene | Beverage/foam cups, toys, window in envelopes |
 | All other resins, layered multimaterials, some containers | Some ketchup bottles, snack packs, mixture where top differs from bottom |
References
American Chemistry Council. “History of Polymers and Plastics for Teachers.”
Katz, David A.: Polymers
University of Southern Mississippi, Department of Polymer Science: Macrogalleria