When it first entered the public consciousness in 1938, nylon claimed a novelty no other product could match. Its predecessor, rayon, had been touted as “artificial silk,” a phrase that implied both economy and imitation. But nylon was billed by its manufacturer, DuPont, as a thing unto itself. As the first commercially viable synthetic fiber, nylon ushered in a fashion revolution based on comfort, ease, and disposability. Its strength, elasticity, weight, and resistance to mildew helped the Allies win World War II. Behind the scenes the invention of nylon also transformed the chemical industry by proving that the composition of polymers could be predicted and engineered like many other chemical products. Today nylon—in toothbrushes, carpet, racket and guitar strings, surgical sutures, car parts, and, of course, hosiery—is all around us.
A Pure Discovery
The first venture of E. I. du Pont de Nemours and Company into artificial fibers came in 1920 when it purchased a 60% interest in Comptoir des Textiles Artificiels, a French rayon company, for $4 million. The combined firm, named the DuPont Fiber Company, eventually became the Rayon Department of the DuPont Company. Although rayon proved popular and profitable, the company expended considerable resources in improving the brittle fiber’s texture and performance—in 1934 alone the company spent $1 million on rayon research.
In December 1926 Charles M. A. Stine, the director of DuPont’s Chemical Department, circulated a memo to the company’s executive committee that suggested the committee was looking in the wrong place for innovation. Rather than investing in practical research directly related to such existing products as rayon or ammonia, Stine argued, DuPont should fund “pure science work.” This work would be centered on “the object of establishing or discovering new scientific facts” instead of research that “applied previously established scientific facts to practical problems.” Stine’s proposal was not new to industry—both General Electric and Bell Telephone operated industrial research laboratories—but his insistence that the research be “pure” or “fundamental” was a fairly radical idea for a company focused on profits. Nevertheless, the executive committee approved a slightly modified version of Stine’s proposal in March 1927. Stine was granted $25,000 a month for research and was told to hire 25 of the best chemists he could find. The committee also approved funds to build a new laboratory, soon dubbed “Purity Hall” by DuPont chemists.
Stine encountered much more difficulty in attracting chemists to DuPont than he had anticipated, largely because academic scientists doubted whether they would truly be allowed to do pure research in an industrial setting. A year later, however, he made a spectacular hire when he convinced Wallace H. Carothers, a young organic chemistry lecturer at Harvard University, to join DuPont. Carothers proposed to center his research on polymerization, the process by which individual short molecules form long-chain macromolecules. Before Carothers’s groundbreaking work most chemists based their polymers on complicated “recipes” largely determined by chance. Moreover, the nature of polymers was poorly understood, with some researchers convinced that the sticky resins represented complex colloidal systems, while others advocated the long-chain molecule theory originally advanced by Hermann Staudinger, a German chemist. Carothers hoped to offer definitive proof of Staudinger’s theory by constructing polymers from small organic molecules with known reactivity at both ends.
Carothers’s success was almost immediate. In April 1930 Julian W. Hill, a research associate in Carother’s group, produced a long polymeric ester with a molecular weight of more than 12,000 by combining a dialcohol and a diacid—this was the first “polyester.” Hill’s polyester fibers had a remarkable property: when cooled, the thin, brittle filaments could be pulled into an elastic thread four times their original length. DuPont researchers soon realized, however, that this first polyester would never succeed as a commercial fiber because its low melting point made laundering and ironing impractical.
For the next four years attempts to create commercially viable synthetic fibers were stymied by the twin problems of low melting points and high solubility in water. In 1934 Elmer Bolton, the new chemical director at DuPont, urged Carothers to return to the problem. Carothers agreed, but this time he would focus on polyamides rather than polyesters. On 24 May 1934 a member of his research team, Donald D. Coffman, successfully pulled a fiber of a polymer based on an aminoethylester. His fiber—ultimately the first nylon—retained the remarkable elastic properties of the polyesters but lacked their drawbacks. However, since the intermediate used to form the polymer, aminononanoic ester, was tremendously difficult to produce, Carothers and his associates kept looking.
Within a year Carothers’s six researchers had narrowed the field to two possibilities: polyamide 5,10, made from pentamethylene diamine and sebacic acid; and polyamide 6,6, made from hexamethylenediamine and adipic acid. (The molecules are named for the number of carbons in the starting materials.) Carothers preferred 5,10, but Bolton pushed for 6,6 because the intermediates could be more easily prepared from benzene, a readily available starting material derived from coal tar. As Carothers’s declining mental health kept him increasingly absent from the laboratory, Bolton’s choice prevailed, and all hands turned to improving fiber 6,6.
Joseph Labovsky, a chemical engineer working as a technician in the lab, later recalled that the lab workers were scaling up fiber 6,6 “from 1 ounce to 1 pound, 2 pounds, 50 pounds, 250 pounds, and eventually to 2,000 pounds.” Paul Flory, a young physical chemist who would later win the Nobel Prize in Chemistry for his work on polymers, helped the researchers stabilize the reaction by developing a mathematical model for the kinetics of the polymerization reaction. In 1938 DuPont started construction on a nylon production facility in Seaford, Delaware, that could produce up to 12 million pounds of the synthetic fiber a year. It was time to introduce nylon to the American public.
On the Market
Nylon’s characteristics made for an ideal material to suit any number of uses, but DuPont decided early on that it would focus on a single market: ladies’ full-fashioned hosiery. As hemlines continued to rise throughout the 1930s, silk and rayon stockings had become an increasingly necessary part of every woman’s wardrobe. American women bought an average of eight pairs of stockings per year, earning Japanese silk producers over $70 million annually. DuPont never intended to produce the stockings directly; rather, the company would provide nylon thread to mills that would knit and sell the hosiery.
As hemlines continued to rise throughout the 1930s, silk and rayon stockings had become an increasingly necessary part of every woman’s wardrobe.
Before DuPont could take its new miracle fiber to the public, however, its leaders had to decide what to call it. In-house researchers had alternately been referring to what would become nylon as Rayon 66, Fiber 66, or “Duparon,” a creative acronym for “DuPont pulls a rabbit out [of] nitrogen/nature/nozzle/naphtha.” In 1938, through a decision-making process that remains somewhat obscure, the company settled on the word nylon. According to Ernest Gladding, manager of the Nylon Division in 1941, the name had originally been “Nuron,” which not only implied novelty but cleverly spelled “no run” backwards. Unfortunately, Nuron and other closely related words posed trademark conflicts, so the division proposed “Nilon.” Changing the i to a y removed any ambiguity surrounding pronunciation, and “nylon” was born. The company then decided not to trademark the name, hoping instead to encourage consumers to think of nylon as a generic preexisting material, like wood or glass.
Since 1931, when Carothers first reported on his polyester fibers at an American Chemical Society meeting, newspapers had been reporting rumors that DuPont had developed a new fiber as good as or better than silk. By early 1938 the press was producing a steady stream of articles that suggested that stockings made from the mystery fiber would outlast silk and never run. If DuPont executives had begun to grow nervous about unrealistic expectations, they grew truly alarmed in September 1938 when the Washington News ran a story based on the newly released patent (U.S. 2,130,948). The article claimed that nylon could be prepared from cadaverine, a substance formed during putrefaction in dead bodies. When combined with reports of Carothers’s suicide earlier that year, coverage of nylon took on an oddly morbid tone. Perhaps to counteract these rumors, for many years thereafter DuPont’s publicity department stressed that nylon was derived solely from coal, air, and water.
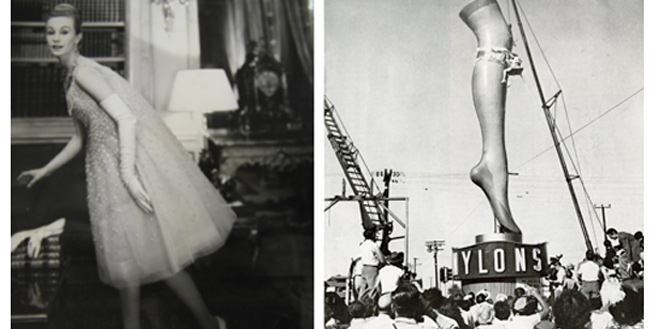
DuPont regained control of nylon’s publicity on 27 October 1938, when it officially introduced the stockings to a crowd of 4,000 enthusiastic middle-class women at the future site of the New York World’s Fair. But while the excitement was building, the stockings themselves would not become commercially available for another 18 months. At that point the only women who could experience the stockings firsthand either worked for DuPont or were married to DuPont scientists in the nylon division. A limited supply of the first pairs went on sale in Wilmington, Delaware, in October 1939, but the stockings did not reach the national market until 15 May 1940. Offered at $1.15 a pair, they were sold out at most locations by noon. In 1940 DuPont produced 2.6 million pounds of nylon, making a total sales figure of $9 million; the following year the company sold $25 million worth of nylon yarn. Within two years of nylon’s introduction DuPont had captured an astonishing 30% of the full-fashioned hosiery market.
The liberal access to nylon hosiery that American women enjoyed proved short-lived. In November 1941 DuPont shifted its nylon manufacture from consumer to military production as a replacement for Japanese silk: in 1940, 90% of DuPont’s nylon had gone into stockings, but by 1942 virtually all nylon went into parachutes and tire cords. Nylon would eventually be used in glider tow ropes, aircraft fuel tanks, flak jackets, shoelaces, mosquito netting, and hammocks. In light of tremendous consumer demand, nylon inevitably found its way onto the black market; one entrepreneur made $100,000 off of stockings produced from a diverted nylon shipment.
Everywhere the stockings appeared, newspapers reported on “nylon riots” in which hundreds, sometimes thousands, of women lined up to compete for a limited supply of hosiery.
DuPont jumped back into consumer nylon production almost as soon as the war ended, with the first pairs of stockings returning to stores in September 1945. Everywhere the stockings appeared, newspapers reported on “nylon riots” in which hundreds, sometimes thousands, of women lined up to compete for a limited supply of hosiery. Perhaps the most extreme instance occurred in Pittsburgh in June 1946, when 40,000 people lined up for over a mile to compete for 13,000 pairs of nylon stockings. Labovsky recalled that demand remained so high throughout the 1940s that DuPont required all its customers, no matter how large or reputable the account, to pay in advance: “The demand was so great. We had to make sure customers who wanted nylon had the money to pay for it . . . Even Burlington Mills would send a check for $100,000 to fill an order . . . Everybody wanted nylon.” Partly in order to meet demand and partly to avoid an antitrust suit, DuPont finally licensed nylon to outside producers in 1951.
Always in Fashion
Nylon stockings represented only the beginning of what would soon become a fashion revolution. Cheap and colorful, synthetic fibers offered the promise of an easy-care, wash-and-wear, disposable future. By the 1950s nylon and other synthetic fibers could be found in underwear, socks, petticoats, fake fur coats, mock-wool sweater sets, and even men’s drip-dry suits. Women’s fashion was especially transformed by synthetic fabrics, as new Lycra girdles—more comfortable and lightweight than traditional rubber models—cinched women’s bodies into dramatic hourglass figures that could then be surrounded with yards and yards of billowing synthetic material.
Because the variety of synthetic fibers was basically limited to viscose (rayon), acetates, polyesters, and polyamides, manufacturers realized early on that the key to their success lay in branding their specific products as unique. Generic DuPont nylon was soon joined in the marketplace by Bri-Nylon, Dacron (polyester), Terylene (polyester), Crimplene (polyester), Orlon (acrylic), Acrilan (acrylic), Tricel (acetate), and seemingly dozens more. Each of the chemical companies making these products then launched extensive advertising campaigns aimed at winning consumers’ loyalty to a branded fabric rather than to the specific fashions of a given season.
DuPont developed a particularly sophisticated approach to marketing its synthetic fibers. From the earliest days of its rayon production DuPont realized that if it was to capture the textile market it needed to capture the hearts of Parisian couturiers. The company’s Fabric Development Department, established in 1926, worked with designers to produce sample fabrics for textile mills and clothing manufacturers. By the mid-1950s the group was producing well over 1,000 fabric samples each year. DuPont salesmen then attempted to sway fashion designers by providing them with generous samples and free publicity. Their first dramatic success occurred at the 1955 Paris fashion shows, in which at least 14 synthetics featuring DuPont fibers appeared in gowns from Coco Chanel, Jean Patou, and Christian Dior. To heighten the glamour DuPont recruited fashion photographer Horst P. Horst to document designers’ works and then circulated the photographs in press releases across the country. Besides couture by Chanel, Dior, and Patou, Horst’s photos featured gowns by Madame Grès, Maggie Rouff, Lavin-Castillo, Nina Ricci, Emanuel Ungaro, Philippe Venet, Pierre Cardin, and the New York Couture Group, all in DuPont fabrics. A decade later, vanguard 1960s designers Pierre Cardin and André Courrèges embraced the futuristic feel of synthetics as the right look for Space Age living.
By the late 1960s synthetics had moved firmly off the runways and into the mass markets—and therein lay their downfall. Victims of overexposure, nylon and polyester seemed suddenly out of date, and their shiny luster started to look tacky. In the wake of Rachel Carson’s Silent Spring (1962) and a growing environmental movement, consumers were turning to natural fibers, particularly cotton and wool. In 1965 synthetic fibers made up 63% of the world’s production of textiles; by the early 1970s that number had dropped to 45%. Although synthetic fibers regained some of their popularity in the 1990s as technical innovations improved their feel and performance, never again would synthetic fibers dominate the market as they did in the 1950s and 1960s.
Yet nylon is here to stay. We may not be wearing it as much, but in one form or another nylon surrounds us in our homes, offices, leisure activities, and transportation. The polymer revolution ushered in by nylon’s discovery has left us with a world of plastics that would be unrecognizable to our grandparents’ generation. Today manufacturers worldwide produce around 8 million pounds of nylon, accounting for about 12% of all synthetic fibers. Nylon may no longer be DuPont’s most profitable product, but it remains one of its most important inventions.
This article has been excerpted from Molecules That Matter, a forthcoming compilation of essays to accompany the traveling exhibit by the same name, opening at CHF’s Clifford C. Hach Gallery in August 2008.




