In the 1850s the new science of organic chemistry was a fertile field ready for growth. But Europe’s chemists found themselves in a quandary. With no standard chemical language and several atomic theories competing for dominance, emerging scientific research became impossible to navigate, and progress stagnated. It took a young, almost unknown outsider to envision a way out.
Stanislao Cannizzaro (1826–1910) was a professor of chemistry in a run-down lab at the University of Genoa at a time when Italians were considered to be outside the scientific mainstream. Despite these handicaps he made a name for himself early in his career with work on organic chemical reactions. His teaching approach was unusual, as he relied on the mostly ignored atomic theories of Amedeo Avogadro, which had been published almost a half-century earlier. Most chemists dismissed Avogadro’s claim of equal volumes of gas containing equal numbers of molecules because it appeared to contradict then-accepted theories on diatomic molecules. In his lectures Cannizzaro showed how Avogadro’s theories could be universally applied to produce a set of standard atomic weights. In 1858 he published a pamphlet on the subject and sent it to fellow chemists, most of whom ignored it.
In 1859, fed up with their students’ struggle to navigate chemistry’s lack of standardization, three chemists—Friedrich August Kekulé, Charles-Adolphe Wurtz, and Carl Weltzien—took action. They resolved to invite Europe’s best chemists to Karlsruhe, Germany, to hammer out their differences and agree on a set of rules and a language. After gaining the support of prominent chemists the three sent out letters inviting all working chemists to join them at the Karlsruhe Congress to develop common definitions for the terms “atom, molecule, equivalent, atomic, [and] basic,” as well as a “uniform nomenclature.”
Unfortunately, sloppily run discussions regularly went off-topic, and most attendees refused to part with their own pet theories. Cannizzaro, the final speaker at the Congress, explained his ideas to a largely tired and uninterested crowd who likely wished only to return home. The conference ended with a vote on whether to adopt the widely known electrochemical theories of Jöns Jakob Berzelius, whose ideas—grounded in inorganic chemical theory—were unsuited to dealing with organic molecules. The split vote ensured that chemists would continue to use whichever nomenclature they preferred.
After the conference’s official end one of Cannizzaro’s colleagues passed out copies of his pamphlet, Sunto di un Curso di Filosofia Chimica, and opinions began to sway. Some attendees were immediately converted; others gave it only passing consideration. Either way, the ideas in the pamphlet began spreading through the scientific community.
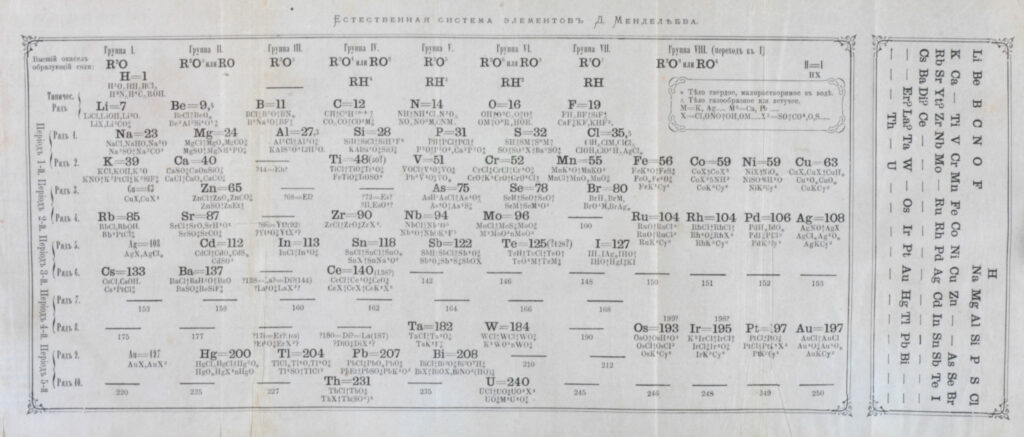
In 1864, Congress attendee Julius Lothar Meyer incorporated Cannizzaro’s ideas into his textbook Die Modernen Theorien der Chemie. Five years later Dmitri Mendeleev presented an early version of his periodic table—now found in every chemistry classroom in the world—to the Russian Chemical Society. Mendeleev, only 26 years old when he attended the conference in Karlsruhe, made no attempt to hide the fact that his table, based on a common set of atomic masses, was inspired by Cannizzaro’s work. A long-overdue consensus within the chemistry community was finally reached.




