In the spring of 1961 the southern and western United States was just starting to recover from a nearly decade-long drought. In towns such as Freeport, Texas, a small port city on the Gulf of Mexico, water rationing prevented people from watering plants, washing cars, or running evaporative coolers (an early form of air conditioning). Nearly everything green had died, leaving yards full of loose, brown dirt that blew away in the breeze. All the while the gulf and its endless expanse of undrinkable water lapped at Freeport’s shore, wordlessly taunting the thirsty town.
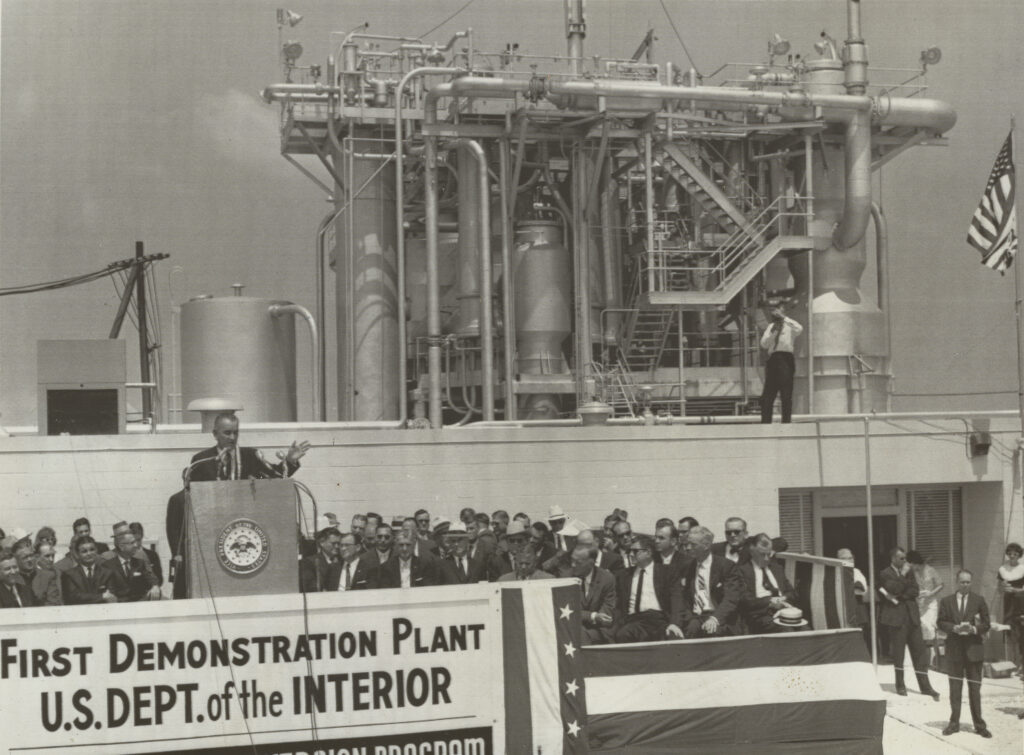
But others looked out to sea and saw a solution. A group of engineers, government officials, and executives at Dow Chemical, Freeport’s most prominent employer, had been working on a scheme to turn seawater into drinking water. Their plan came together in 1961 with the opening of a brand-new desalination plant, the first of its kind in the United States. The event was a big enough deal to command the attention of the U.S. president. On June 21, the first day of summer, Vice President Lyndon Johnson was sent to Texas to preside over a dedication ceremony. John F. Kennedy kicked off the celebration with a speech for the families and reporters gathered outside the plant.
Kennedy, still dealing with the fallout from the failed invasion of Cuba and a fruitless negotiation with Nikita Khrushchev over the status of a divided Berlin, did not travel to Freeport that day. Instead a camera crew recorded his speech at the White House, sending the audio by telephone to the crowd in Texas.
Conjuring the same tone used a month earlier to declare his ambition to put a man on the moon, Kennedy announced that desalination “is a work that in many ways is more important than any other scientific enterprise in which this country is now engaged.” It would allow the deserts to literally bloom, he claimed, and would help raise men and women out of poverty.
When Kennedy finished his speech, he reached over and pushed a shiny black button on his desk. “I hope it worked,” he said, smiling awkwardly. Back in Texas a spigot, supposedly triggered by Kennedy’s button, shot forth a stream of fresh water that gushed into a tank and quickly overflowed. Newsreels of the speech made Kennedy’s message clear: with desalination no one would want for water again.
Water covers about 70% of the planet, but only 2.5% of that water is fresh, and only about half of that fresh water is accessible. That lopsided ratio means for millenia humans have been encountering the same irony memorialized by Coleridge: “Water, water, every where, / Nor any drop to drink.” Aristotle studied the conundrum around 340 BCE, observing that “salt water, when it turns into vapor, becomes sweet, and the vapor does not form salt water again when it condenses.” However, the philosopher never suggested such a process should be used to create drinking water; he was merely describing a part of what we now call the water cycle—the constant evaporation, condensation, and precipitation of H2O.
Until the 20th century practical examples of desalination were mostly limited to seafaring expeditions. In 1603 English explorer Richard Hawkins wrote of supplying his crew with distilled seawater during his travels in the Pacific. World War II battleships and submarines on both sides were outfitted with small seawater-purification machines to help keep their crews hydrated. After the war such machines were commonly used to extract minerals, such as lithium, from solutions or to make products through dehydration, including maple syrup, sugar, and condensed milk.
As the world population exploded in the 20th century, especially in places without enough water to support such growth, large-scale desalination started to look more appealing. A handful of seawater evaporators were installed on the bone-dry Caribbean island of Aruba between the 1930s and the 1950s to provide drinking water for workers at oil refineries, then the island’s primary industry. In 1951 the desert nation of Kuwait invested in its first evaporator-based desalination plant. Despite these examples few people in dry coastal areas of the United States were thinking about distilling salt water at this time, but that changed when the worst drought in the nation’s history struck.
Between 1949 and 1951 the annual rainfall in Texas decreased by about 40% and averaged just three-tenths of an inch per month by 1953. Summers reached record high temperatures, and dust storms ravaged the Texas Panhandle and bordering states, with one Amarillo newspaper reporting that the city was in a “complete blackout” in the middle of the day from the amount of sand in the air. While towns such as Freeport struggled to get by on limited water, ranchers and farmers were hit hardest. In 1953 the combined income of Texan farmers dropped by one-fifth from the previous year. Farmers and ranchers were forced to abandon the cracked land and sell off their cattle. The drought also scrambled the state’s demographics: at the beginning of the drought more than a third of all Texans lived in rural areas; by the end only a quarter of the state’s population was rural.
Congress had already gotten to work on the problem. In 1952, as the drought affected more states, including Oklahoma and New Mexico, lawmakers passed the Saline Water Act at the urging of the secretary of the interior, Oscar Chapman. If rain was not going to come to the suffering states, then scientists would have to summon water themselves.
Large-scale desalination technology was still in its infancy, and the American program was originally allocated a mere $2 million over five years. By 1955, with the drought worsening, Congress established the Office of Saline Water, quintupled funding for the effort, and in 1958 tasked the new office with opening five demonstration desalination plants around the country. Each plant would be built in a different region (those not near the coast would draw brackish water from the ground), and each would use a different technology; the most efficient and successful plant would, it was hoped, pave the way for dozens more like it. Freeport, Texas, was chosen as the first location to receive a demonstration plant, partly because the town’s main employer, Dow, already owned a conveniently located patch of land and was ready to buy a percentage of desalinated water for factory use.
Texas senator Lyndon Johnson witnessed the impact of the drought on his constituents. Having also experienced the Dust Bowl of the 1930s, he could see that drastic action was needed to secure reliable sources of water. During his time in the Senate, Johnson worked on water resource reform, including building dams that saved fresh water in reservoirs, and put his full backing behind legislation to support the U.S. desalination program. Johnson continued to push Congress for funding and spoke passionately about desalination while campaigning as Kennedy’s running mate in 1960. Writing for the New York Times Magazine a week before the election, he asserted that “probably no group anywhere has done more [research on desalination] in the last eight years than the United States Office of Saline Water.” He praised the work of the office’s staff while bemoaning the “pathetically little money” provided for research and emphasizing the importance of convincing other legislators to support future desalination research.
Meanwhile the demonstration plant in Freeport needed to be built. In 1957 the House of Representatives had summoned a handful of experts to consult on the technology to be used in the proposed plant. Among those called was Walter L. Badger, a chemical engineer and former University of Michigan professor with a reputation as a demanding, derisive teacher. (Badger was notorious for calling students to the blackboard to sketch out evaporator designs while he blew smoke in their faces and hurled insults.)
At 72 years old, Badger had hardly mellowed. He pitched the committee an audacious plan for a one-million-gallon-per-day desalination plant built with then cutting-edge evaporator technology of his own invention. Badger was exceptionally qualified for the job and for making such a bold proposal: he had founded an evaporation laboratory at the University of Michigan, had forged a unique research partnership between the university and a private evaporator company, had established his own consulting firm that worked with clients from all over the world, and had published more research on evaporators than anyone else. “Consequently,” Badger told the legislators, “I feel I have some right to talk about evaporators.”

Caricature of chemical engineer Walter Badger from the 1931 National Meeting of the American Chemical Society.
Badger believed his technology would allow him to get the plant operational within a year of starting construction, a staggeringly short time for a project of such scale. Badger’s swagger won over the lawmakers, and his company, W. L. Badger Associates, was offered the contract.
Badger dubbed his technology long-tube, vertical, multiple-effect evaporators. That convoluted term boils down to one simple idea: steam generated from evaporating salt water in one chamber can be used to heat up another chamber of salt water. Then the steam generated in the second chamber, can heat up the salt water in a third chamber, and so on. (The Freeport plant was designed with 12 chambers.) At each stage the steam cools and some of it condenses into pure water, which can be pumped out as clean drinking water. Badger also theorized he could offset energy costs if he installed turbines to harness the steam to generate electricity.
If Badger’s evaporator technology sounds a lot like Aristotle’s method from more than 2,000 years before, that’s because it’s founded on the same basic chemistry: when salt dissolves in water, the water molecules ease apart the salt’s chloride and sodium ions just enough to wrap each ion in tiny bundles of liquid H2O. Heating the salt water overpowers the forces holding the ions in place, allowing the sodium and chloride to latch back together and pure water to escape as steam. On an industrial scale this process requires significant energy, so Badger’s technology needed to be as efficient as possible.
Within a few months of beginning the project, Badger ran into a major setback. His previous evaporation work had mostly involved taking minerals out of solutions that were less corrosive than seawater. While testing a prototype evaporator he realized seawater deposited a large amount of salty gunk (known as scale) on the insides of the pipes, which slowed water flow and corroded the metal. He admitted to Congress, “There is a lag here . . . that nothing but just plain elbow grease will lick.” Badger tested different alloys until he found one that resisted the buildup, and he devised a system to maintain a high level of acidity in the water, which he claimed would reduce the scale problem. His vertical-tube configuration meant gravity would also help keep scale at bay. With the problem solved, Badger was confident the plant would still be ready by the time he promised. Then he suddenly died.
Its eponymous leader was gone, but W. L. Badger Associates still managed to complete the project on time. By the summer of 1961 the plant was finished; it consisted of a dozen eight-story-high chrome canisters connected by tubes and scaffolding and was powered by a coal-burning furnace located at a nearby Dow factory. The plant was an energy hog, but it could pump out a million gallons of fresh water each day for the citizens of Freeport and for Dow’s operations.
The Freeport plant was not just an engineering triumph for the late Badger and his company; it was also a political win for Johnson, who as senator and vice president had lobbied the politicians in charge of the government’s purse strings. At Johnson’s urging Kennedy used the spectacle to sell the public on the government’s desalination program. While Kennedy was broadcasting his speech to Freeport from the White House, Johnson oversaw the unveiling in person, promoting water conservation and explaining the plant’s inner workings to the crowd.
Kennedy’s button press, unsurprisingly, was just for show: plant workers had quietly been mixing desalinated water into the regular supply and pumping it into Freeport homes for more than a month. No one in Freeport was ever the wiser, and when they found out, wrote the New York Times, “there was no complaint.”
After the success of the Freeport plant four more demonstration plants—each featuring a different technology—were built around the country within a few years. Badger’s company was chosen to design and engineer a plant near Wilmington, North Carolina, that would use freezing instead of evaporation to purify water. (When ice forms on the surface of water, its crystalline structure pushes salt and other minerals away. When the ice is melted, the resulting water is drinkable.) The program seemed particularly prescient when drought struck the normally rainy Northeast between 1961 and 1969. In the face of increasing water shortages these plants proved to Americans that large-scale desalination was feasible (if expensive) and the country could look forward to a future where it would no longer be at the mercy of the weather. Science, it seemed, had again triumphed over nature. Then the rain came back.
Rain began returning to the Southwest at the end of the 1950s. By the time all the demonstration plants were completed, the Texas farming industry was growing again, and Freeport’s reservoirs were full. The water produced by the Freeport plant, which cost the government as much as $1.25 per thousand gallons to produce, was sold to the town of Freeport for 30 cents per thousand gallons, a price consistent with naturally sourced water in other parts of the country. Without the pressure of a drought Congress had little reason to continue such subsidies.
In 1969 funding for the Freeport plant ran out, and it ceased operations. Plans were scrapped for another, bigger plant near Los Angeles—to be built on an artificial island and fueled by nuclear energy—that could produce 150 million gallons per day. In 1974 the Office of Saline Water was officially rolled into the Office of Water Resources Research, which was focused on a multitude of environmental and agricultural issues, including protecting lakes, rivers, and other sources of fresh water. Over time desalination became less of a priority, and the cost of running a desalination plant became even less appealing when the energy crisis of the 1970s arrived. The Office of Water Resources Research was abolished in 1982 as part of President Ronald Reagan’s effort to slash most non-military, government-funded science programs. The emaciated desalination program was eventually reestablished under the Office of Water Policy and was ultimately moved to the U.S. Geological Survey in 1984, at which time Congress had to override Reagan’s veto to continue funding it.

But even as the United States was curtailing desalination research, other countries with less reliable water sources were starting to invest. Some, such as Kuwait and Qatar, had already gone down that path. Kuwait’s first evaporation-based desalination plant was commissioned in 1951, a full year before the United States passed the Saline Water Act. The small plant pumped a little less than 10,000 gallons of purified water from the Persian Gulf to Kuwait City every day. In 1953 Kuwaitis built a new plant that eventually reached a capacity of about 2.5 million gallons per day. In the years that followed, the country continued to invest in desalination, building plants with ever newer technologies. The breakneck pace of Kuwait’s desalination program can be largely attributed to the country’s unique needs and resources: there are no permanent rivers, and more water evaporates from the land each year than is replenished by rain. But what Kuwait lacks in water, it makes up for in oil. Rather than import water from other countries Kuwait’s government decided to export oil and use the resulting wealth to make its own water. By the early 1960s Kuwait was desalinating nearly 6 million gallons of water each day.
Early proponents saw desalination as a technology that could pull the postwar world together. Johnson wrote in his 1960 article for the New York Times Magazine, “We must draw together and stimulate the scientific effort of the entire free world and center it, with all the necessary resources, upon conversion of salt water.” Johnson believed providing clean water to parts of the world that didn’t have it would not only alleviate suffering but would create political alliances and bring peace to fractured regions, especially in the Middle East. Invoking the Atoms for Peace program that sought to share nuclear energy with friendly nations, Johnson announced a Water for Peace program that would work much the same way with desalination technology. The two programs had more in common than just a name; at one point U.S. officials made tentative plans to help Israel build a handful of nuclear-powered desalination plants. The cost of the proposed partnership ultimately proved too much for the American government. Still, Israel pushed on with its desalination program and opened its first plant in 1964 in Eilat, a desert town on the Red Sea. The plant used a freezing process to separate salt from water, similar to the Office of Saline Water’s pilot plant in North Carolina. Israel’s desire for water independence was motivated not just by the natural water scarcity of the Middle East; it was also driven by politics. There was no guarantee neighboring states would honor any agreements to share the Jordan River, one of Israel’s few sources of fresh water. (In fact the Israeli government had approached Badger in 1958 about consulting on a dual-purpose electric power and water desalination plant even before Johnson’s Water for Peace program existed, but Badger never fully committed to the invitation. At the time, Badger was selling evaporators to Arab nations, and he didn’t want to damage his relationship with those countries.)
Soon the other Gulf nations also began investing heavily in desalination. Following the example of Kuwait they decided to use the vast wealth generated from oil to solve water shortages. Saudi Arabia created the government-owned Saline Water Conversion Corporation in 1965 and shortly thereafter began opening desalination plants, which have since been sustained by the West’s endless appetite for oil. (Desalination was not the only Saudi scheme to acquire fresh water. One Saudi prince proposed towing an iceberg from Antarctica to the Persian Gulf, but the half-baked plan never took shape.)
Most of the desalination plants in the Middle East were based on the same evaporation technology pioneered by Badger. But in addition to evaporation and freezing, the Americans were working on a third type of technology that would turn out to have the most promise for the future: membranes.
Badger’s evaporators at the Freeport plant used loads of energy from coal-fired furnaces to force salt and water apart, but a demonstration plant in Webster, South Dakota, which drew brackish water from the ground, employed semipermeable membranes that took the salt out of the water through a process called reverse osmosis. Essentially, the water was pushed through a series of barriers with openings of a size and shape that only water molecules could pass through, leaving behind most salt and other impurities.
Like the other pilot plants, the Webster operation was shut down because of lack of funding, but by the 1980s membranes had become the standard technology used in many of the world’s desalination plants. Today further advancements in nanotechnology are making the membranes smaller and more effective at filtering out unwanted particles, and evaporation and distillation plants are now in the minority.
Israel, Saudi Arabia, the United Arab Emirates, and Australia now obtain a significant percentage of their water from ocean-fed plants that push water through membranes. Kuwait and Qatar are entirely dependent on desalinated water for both domestic and industrial uses and spend billions of dollars each year to keep it flowing. In 2015 the International Desalination Association estimated that around the world there are more than 18,000 desalination plants providing water to more than 300 million people. Currently, that desalinated water only provides about 1% of the world’s drinking water, but the International Water Association expects production capacity to double by 2030. It may need to grow even faster to keep up with climate change.
A paper published by the American Meteorological Society predicted that the total percentage of Earth’s land in extreme drought at any given time will have grown from 1% in 2006 to 30% by the end of the 21st century. As climate change redistributes water around the planet, causing wet places to become wetter and dry places to become drier, more and more people are going to be dependent on alternate water sources. Even the United States, where droughts have again increased during the past two decades, has begun reinvesting in desalination. A number of membrane plants have come online in Texas and California since 2005, and others are planned for Los Angeles and Corpus Christi.
Beyond the energy consumption and expense, desalination has another major downside: it produces salt—lots and lots of salt. Some companies have found ways to monetize the dried effluence as sea salt, but most of that extra-salty brine sinks to the sea floor where it kills marine life.
Concentrating more salt into a body of water also makes it more difficult to extract water from it in the future; the more salt in the water, the more energy it takes to filter it out. Almost every country bordering the Persian Gulf now relies on desalination for drinking water, and together those countries deposit millions of gallons of concentrated brine into the sea every day. A 2012 study by scientists in the United Arab Emirates found that brine discharged from desalination plants into the Gulf caused the salinity of nearby water to rise from an average of about 45 parts per thousand (that is, for every kilogram of water there are 45 grams of salt) to 50 to 55 parts per thousand. Eventually, the Gulf could become so salty that not only will it be impossible for fish to breathe, but it will be too expensive to justify extracting fresh water. This phenomenon has been dubbed “peak salt.”
Scientists are looking for a solution. A chemical engineer at Qatar University patented a process to divert nearly 100% of the brine discharged from Gulf desalination plants and convert it into baking soda and calcium chloride (often used to de-ice roads), which can then be sold. Even if these schemes are successful, such plants would still have enormous carbon footprints. To address pollution concerns Saudi Arabia and other countries, especially Australia, are building solar-powered desalination plants.
Critics of desalination argue that it’s wasteful to spend money and energy on desalinating seawater (even if the plants are powered by solar panels) when humans dump billions of gallons of wastewater into lakes and rivers every day. Why not recycle the leftover water from showers and toilets and send it back into the system instead of wasting energy desalting the relatively dirtier ocean water? Part of the reason some governments have been resistant to recycling water is because the concept sounds a little gross; detractors call it “toilet to tap.” Proponents point out that water released from treatment plants is cleaner than the water drawn from lakes and rivers. And they add that we already use a toilet-to-tap system: it’s called the water cycle. The water taken from rivers and lakes once filled the toilets of municipalities upstream. However, according to the United Nations, around the world there are at least 1.2 billion people living in areas that don’t have water to recycle to begin with. For those people the only options appear to be desalination or importing the resources they lack.
With climate change causing more frequent droughts, desalination offers a potential solution to sustain growing populations in the most arid regions of the planet. However, investment in the technology has been almost entirely reactionary; Badger’s plant in Freeport was decommissioned in response to the drought ending, but now there are plans to build a completely new desalination plant just a few miles away from the original. Will that plant be shut down too if Freeport gets a few rainy years?
Brazoria County, which contains the city of Freeport, had a population of fewer than 80,000 residents in 1960. Today that population has swelled to more than 360,000. Traditional water sources, including the Brazos River that cuts across most of Texas and empties into the Gulf of Mexico, are starting to dry up as more and more people upstream divert water for their own needs. By the time the river arrives in Brazoria County, it has been filled with runoff and slowed to a crawl. Residents hope the new plant will offer enough water to offset the losses from the dwindling river. However, construction has been delayed by concerns of building too close to wetlands, a frequent problem when constructing desalination plants. There is limited coastal real estate, and much of what is undeveloped provides ecosystem services, such as flood control, water cleaning, and groundwater restoration.
On the surface, desalination is a concept that can be understood by anyone: use science to turn the seas into drinking water. Perhaps that is why Johnson and Kennedy had such an easy time selling it to a nation in crisis and why it keeps gurgling up wherever climate change threatens water sources.
Of course it’s not that simple: wherever desalination has been implemented, it has extracted huge financial and environmental costs. Some countries, such as those in the Middle East with fast-growing populations, may have no choice but to make that trade and suffer the consequences. For others the ultimate question is how to get water to the people who need it. Desalination is just one answer.