On the morning of November 1, 1952, four jet fighters flew through the skies above Enewetak Atoll, an isolated horseshoe of coral islands in the middle of the Pacific Ocean. In the cockpit of his F-84 Thunderjet, Captain Jimmy Robinson checked the unusual assortment of dials and gauges in front of him. Robinson’s plane had been modified for the day’s assignment: his dashboard was augmented with a suite of scientific instruments, and the cigar-shaped fuel tanks on the wings were refashioned to hold filters to scoop up passing debris. He wore a protective, lead-lined jacket in place of a life preserver.
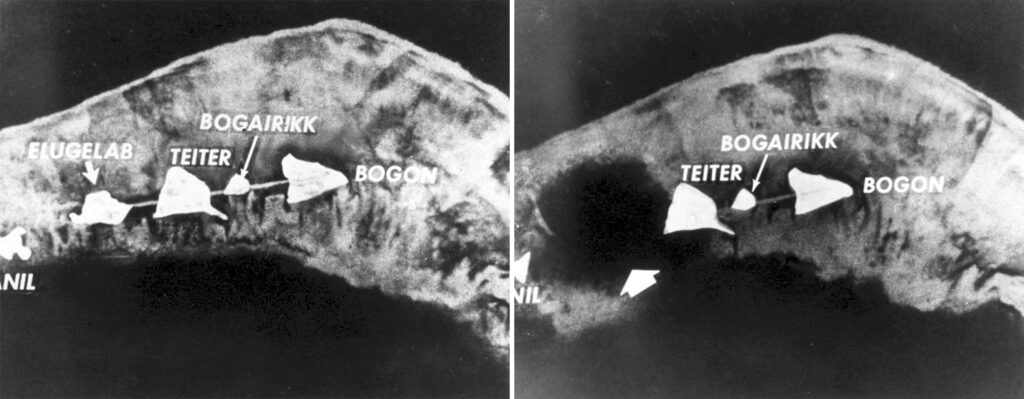
The 28-year-old, who had been shot down over Romania in 1944 and spent 10 weeks as a prisoner of war, was about to undertake the most perilous mission of his career. An hour earlier, at 7:15 a.m. local time, “Mike”—the world’s first hydrogen bomb—had been detonated on the island of Elugelab, on the northern bend of the atoll. Robinson and the rest of his team were heading into the mushroom cloud.
The U.S. military had been using planes, usually staffed with scientists, to sample debris from atomic blasts since the earliest nuclear explosions. The first nuclear test, in July 1945, was witnessed from the air by future Nobel Prize–winning physicist Luis Alvarez (who also witnessed the dropping of the atomic bomb on Hiroshima). Unmanned drones were also flown into atomic clouds to take samples during test detonations, their controllers hoping for insights into the new superweapon. Yet the first manned plane to go into a mushroom cloud did so against orders. On May 14, 1948, a B-29 bomber piloted by Lieutenant Colonel Paul Fackler cut through a finger-like spur of gas that had drifted away from an atomic test. Fackler never said whether the move was an accident or a deliberate stunt, but as he reported later, “No one keeled over dead, and no one got sick. In fact, there seemed to be no undue alarm among the crew members.” The U.S. Air Force decided it had all the evidence it needed to send pilots inside the most challenging environment on Earth.
“Most pilots with less experience and proven ability were simply overwhelmed . . . by the awesomeness of the clouds’ ever-changing interior,” Guthals concluded.
Three groups of planes would pierce the cloud’s interior that morning; the first, made up of four planes, was code-named Red Flight and took off from a remote strip on Enewetak Island, at the far southeast of the atoll that bore the same name. The four pilots were all war veterans who had kept their cool in hostile skies. Their leader, Virgil Meroney, had shot down 10 German aircraft. Robinson, in Red-4, had spent the war as bombardier of Dazzlin’ Dutchess, a B-24 Liberator, before he was shot down over Romania. Parachuting free of the burning wreck, he had calmly lit a cigarette as he drifted back to Earth. After the war he had transferred to fighter jets and had already made a practice run through a smaller atomic blast in Nevada.

Navigating through “Mike” would be another thing entirely. A nuclear bomb uses fission, each atom exploding and releasing neutrons that collide with neighboring atoms, overloading them and causing them to detonate—a chain reaction. But this isn’t a perfect process: much of the potential fuel of the first generation of nuclear weapons was wasted, and so in reality the detonations were significantly smaller than their theoretical yield.
A hydrogen bomb, the brainchild of physicists Edward Teller and Stanislaw Ulam, worked differently. Teller and Ulam added deuterium and tritium—neutron-rich isotopes of hydrogen—to the bomb to generate a two-stage explosion that created an even greater blast. Mike detonated with a force of about 10.4 megatons—around 700 times more powerful than the bomb that had destroyed Hiroshima seven years earlier. A three-mile-wide fireball stretched across the horizon like a second sun and wiped Elugelab from the map, leaving a 164-foot-deep crater in the ocean floor. In less than 90 seconds the mushroom cloud soared above 55,000 feet—the maximum ceiling for the Thunderjet—and eventually spread 25 miles high and 100 miles wide.
That was bad news. The pilots couldn’t fly through the top of the cloud, so they would have to make their run through the stem—a swirling cauldron of unpredictable winds, heat, and radiation that was thick with sand and coral from Elugelab’s remains.
Around 90 minutes after the Mike explosion, Meroney and his wingman ventured inside the maelstrom. Immediately their cockpits filled with a red glow Meroney described as “like the inside of a red-hot furnace.” Battling the winds and raging dust storm, Meroney kept his eye on the three radiation instruments in his cockpit. They spun, he later reported, “like the sweep second hand on a watch.” After five minutes the radiation instruments had “hit the peg”: whatever was in the stem of the cloud was greater than his plane could measure. Meroney broke free of the stem, paired up with his wingman, and ordered Robinson in Red-4 and pilot Bob Hagan in Red-3 to make their run. Meroney radioed across a final warning as the pair disappeared: “Don’t go in too far.”

The maelstrom hadn’t calmed. Hagan radioed that the sky had “grey and dark shades and still appear[ed] to be boiling.” From Robinson there came only heavy breathing. He had hit a pocket of severe turbulence almost immediately on entering the radioactive smoke, making his plane spin out of control. Battling against the yoke, he accidentally hit his microphone, broadcasting his struggles for control and consciousness. Finally, mercifully, he wrestled control of the plane at 20,000 feet. Meroney ordered Robinson and Hagan to exit the cloud immediately and find the tanker plane for refueling. The four men had done all they could.
But Robinson’s and Hagan’s ordeal had only just started. Under the oppressive darkness of Mike’s still-burgeoning cloud, they were unable to spot the tanker; the bomb’s electromagnetic storm had scrambled their instruments, so they couldn’t lock onto the homing beacon from the airstrip at Enewetak. Lost in the Pacific, the two pilots searched for a place to land, but their efforts were hampered by rain squalls and limited visibility. By the time they spotted the field at Enewetak, they were almost out of fuel.
Hagan made his approach first, and with extraordinary skill he glided his plane onto the runway in a dead-stick landing, bursting a tire on impact. Robinson was less fortunate. His engine flamed out 3½ miles short of safety, leaving him no option but to try to land on the ocean surface and hope a rescue helicopter could get to him in time. His plane hit the water, bounced, and flipped. The chopper arrived just as the plane slipped beneath the surface; all that remained was an oil slick, a glove, and some maps.
A year later Robinson was posthumously awarded the Distinguished Flying Cross. He left behind a wife and baby daughter.
But the story of Red Flight was far from over. On landing the survivors went through the standard decontamination procedure. Each pilot was lifted free of the cockpit on a cherry picker, stripped of his gear and clothing, and sent to a decontamination shower. Meanwhile, five servicemen known as the decon-grunts moved in to wash down the planes and pluck out the wing pods’ filters using 10-foot poles. Each filter was then sealed in a lead “pig” and shipped back to scientists at Los Alamos, New Mexico. There the scientific value of the mission began to emerge.
The Mike samples were difficult to handle, highly radioactive and heavily contaminated with calcium carbonate from the coral debris. Even standard chemical tests were hard to perform: simply dissolving a sample in acid could cause it to catch fire. The Los Alamos team was forced to work outside in a purpose-built tent. Despite the unorthodox lab setup the filters soon proved to be a treasure trove. The team found traces of plutonium-246—the most neutron-rich isotope of the element ever seen, as well as plutonium-244, an isotope with a radioactive half-life of 80 million years.
News of the Mike test and its results were classified, but by December word had reached the University of California, Berkeley, where chemist and recent Nobel Prize winner Glenn Seaborg, received a brief report on the Los Alamos findings.
A decade earlier Seaborg had discovered plutonium through neutron capture. Usually, when an atom is pummeled with neutrons, it will explode via fission. But occasionally the atom will capture a neutron, which will radioactively decay into a proton, pushing the element one place up the periodic table. Since World War II, Seaborg’s team had specialized in making radioactive elements too short-lived to be found in nature, extending the periodic table to element 98, californium. But the elemental gold rush had come to an end: the team couldn’t find a source with enough neutrons to make the next element, number 99.
Seaborg took the top-secret report to his colleague Al Ghiorso, the Berkeley team’s technical expert who had been working with Seaborg for a decade despite holding no more than a bachelor’s degree. Ghiorso made a quick calculation: if Los Alamos had found neutron-rich plutonium, the filters might also have captured atoms that had radioactively decayed into the undiscovered elements 99 and 100. If they could get hold of one of the sampling jets’ filters, they could find two more components of the physical universe. “It was the wildest idea of my entire career,” Ghiorso later wrote. Seaborg didn’t believe it possible but gave Ghiorso and his colleague Stanley Thompson permission to try.
The Berkeley team called in a favor from an old colleague and managed to obtain half a filter paper from the Mike test. For the next month the team ran chemical tests and measurements, finally discovering elements 99 and 100 through their telltale radioactive decay into known elements. Almost immediately the Berkeley scientists found their discovery challenged by Argonne National Laboratory researchers in Chicago, who had also received a filter and claimed the discovery for their own. But through scientific argument, politicking, and what Ghiorso and Seaborg described as an “abundance of cocktails,” the Berkeley team eventually persuaded Argonne that the discovery was theirs, shared with the team at Los Alamos. The existence of the two elements remained secret for another two years until a team in Sweden managed to create element 100 in the laboratory. Freed from the shroud of nuclear military secrets, the Berkeley team published their findings showing they had also created element 100 in the laboratory and had discovered the element even earlier through “experiments”—in other words, through the Mike explosion. The original discovery was American made.

In 1955 the two new elements were named einsteinium and fermium, after Albert Einstein and Enrico Fermi, who had both died shortly before the announcement. To date neither element has any practical application, and they can be produced only in a handful of laboratories around the world. They seem a heavy price to pay for a man’s life.
And yet their discovery opened up a new era in nuclear research, which has led to the 118 elements we know today. Other created elements have found roles in treating cancer, providing energy, and powering life-saving devices, such as smoke alarms. Perhaps einsteinium and fermium will one day find their own roles.
As for Robinson he is part of the legacy of those who risked—and sometimes lost—their lives to expand the frontiers of knowledge. Robinson’s own legacy took a long time to be recognized. His death fell into a bureaucratic mess of red tape, with no one sure who was responsible for honoring his memory. He was finally given a headstone at Arlington National Cemetery in 2002, 50 years after his final flight. His widow accepted a flag in memory of his service. Today his daughter, Becky, works with atomic veterans to ensure their stories are told.




