—Raymond Chandler, The Little Sister, 1949
Philip Marlowe, the hard-boiled hero of Raymond Chandler’s detective novels, was right about neon. The inventors of the lights that set the night sky aglow in a thousand cities literally had made something out of nothing. The colorful words and pictures came from the air itself—mysterious gases extracted from the atmosphere, trapped in glass tubes, and zapped with electric current to create luminous reactions. During the 20th century, lights fueled by neon and its fellow noble gases were icons of commerce and entertainment, illuminating the modern age. Some early computers and calculators even used small neon tubes for circuits and displays. Today, many of the large, elaborate neon signs have sputtered out, replaced by newer or cheaper technologies, but these gas-filled tubes still shine on a smaller scale, treasured for their unique light.

Aristocrats of the Air
The story of neon begins in the 1890s, with Scottish chemist Sir William Ramsay. Best known as the codiscoverer of four of the noble gases (neon, argon, krypton, and xenon), Ramsay also isolated and characterized helium and radon, the other two noble gases, winning the Nobel Prize for his efforts. Together, these six gases form a family of elements distinguished by their unwillingness to bond with other atoms. This standoffish “nobility” gave the noble gases their name.
It was a long time before the atmosphere gave up all its secrets. As early as 1785 prominent chemist Henry Cavendish had noted a small residue of gas left over after he removed oxygen (then known as “dephlogisticated air”) and what we know now as nitrogen from “common air.” Ramsay and his mentor John William Strutt, 3rd Baron Rayleigh, took up the challenge of identifying this mystery gas. In 1894 they began attacking air with brute-force methods—combustion, reaction, and absorption—to strip away every possible atom of nitrogen and oxygen. In one early experiment they removed oxygen from air using red-hot copper. To remove nitrogen the deoxygenated air was passed over red-hot magnesium, soda lime, copper oxide, and phosphoric anhydride. After further steps eliminated the remaining nitrogen and oxygen, they named the residual gas argon (derived from the Greek for “the inactive or lazy one”).
Though argon makes up less than 1% of the atmosphere, Ramsay suspected that there were even rarer gases hidden in the air. In the summer of 1898 he and his colleague Morris W. Travers hunted down these additional elements. Starting with purified argon liquefied at low temperatures, they slowly added heat to isolate gases that boiled at temperatures both above and below argon’s boiling point. In this way they discovered neon, krypton, and xenon (Greek for “the new one,” “the hidden one,” and “the stranger”).
Though these rare gases are invisible to the naked eye, each one glows with a distinctive brilliant color when sealed in a glass tube and energized with high voltage. These gas-discharge tubes, named for the electrical discharge that made them light up, would become the basis for neon lamps. Ramsay found neon’s light particularly striking. In his 1904 Nobel Prize lecture he described the neon spectrum as “a brilliant flame-coloured light, consisting of many red, orange, and yellow lines.” Travers was even more effusive:
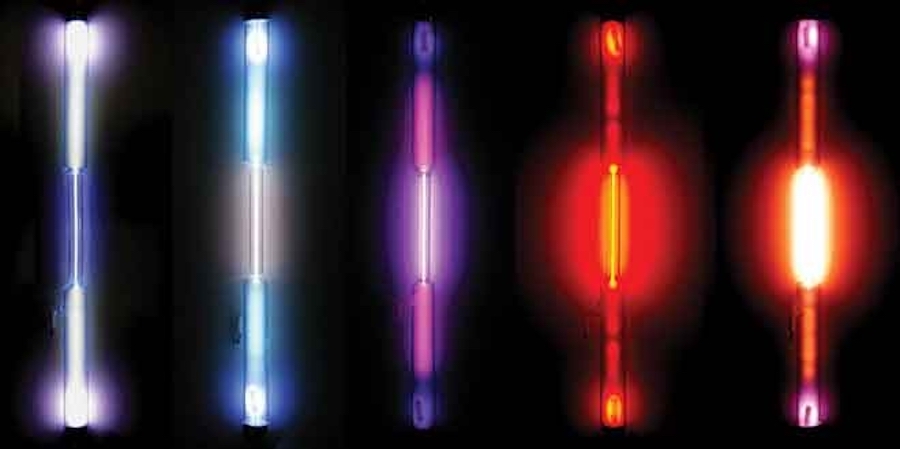
Mining the Air
Beginning in the late 19th century liquid air, particularly oxygen, found many uses, including in theater lighting and industrial welding. All techniques for liquefying gases used the Joule-Thomson effect—seen in the home or office today whenever a can of pressurized air is used to dust a computer keyboard (as the air expands through the nozzle, the temperature drops and condensation forms on the can). The first practical methods to liquefy air on a large scale appeared while Ramsay was working to isolate his gases, and he made sure to thank William Hampson, one of the men responsible, in his Nobel Prize lecture.
Like Hampson in England, Carl von Linde in Germany, and others, Parisian electrical engineer and inventor Georges Claude applied the Joule-Thomson effect to liquefying air, scaling up the process to produce huge quantities (up to 10,000 cubic meters per day). With his former schoolmate and colleague Paul Delorme, he formed a company in 1902 named simply L’Air Liquide that expanded rapidly to become a multinational corporation. While selling liquid oxygen for industrial purposes, Claude carried out scientific research. At first, he had hoped to discover additional noble gases by analyzing large volumes of liquefied air, but he was forced to admit that “after Ramsay there was nothing more to be done.” His next project combined the leftover neon from his liquefaction of air with his dislike of the overwhelming brightness of electrical lighting at the time.
Lines of Light
Claude was not the first to look to gas tubes for light. Spurred by the commercial success of Thomas Edison’s incandescent lightbulbs, inventors attempted to transform gas-discharge tubes into practical lighting systems. In the late 1890s Daniel McFarlan Moore, a former Edison employee, filled 10-foot glass tubes with nitrogen or carbon dioxide under low pressure, adding electrodes at both ends. These “Moore lamps,” which glowed bright white when electrified, were more efficient than the carbon-filament incandescent bulbs then in use. Though the lamps were used as general lighting in some stores and workplaces, they were expensive to install (a “glass plumber” had to connect the tubes on-site), required high-voltage electricity, and tended to leak. After 1910, when improved incandescent lamps with tungsten filaments displaced Moore’s tubes, his company went under.
Claude soon found that adapting Moore’s concept to neon involved more than just switching gases. His tubes gave a magnificent glow, but impurities set free from the hot electrodes quickly dimmed the brightness. A carbon filter solved that problem but not the issue of metallic buildup around the electrodes, which made the tubes flicker out too soon. Claude installed larger electrodes that stayed cooler: the resulting tubes burned brightly and steadily, with 20-foot tubes lasting as long as 1,200 hours.
Successful at last, Claude filed his first patent for neon lighting in 1910. That December he demonstrated his invention at the Salon de l’Automobile (the Paris Motor Show). Inside the exhibition building thousands of incandescent bulbs studded light fixtures and manufacturers’ signs, glinting off the shining metal of the cars below. Outside, two 40-foot neon tubes glowed a vivid orange-red on the building’s colonnade. Modern technology of all sorts was on display: the newest cars and the newest lighting made possible by the electrical network then spreading rapidly throughout Paris.
Claude admitted that red neon was not ideal for general lighting but insisted there were some situations in which neon would prove superior, such as for illuminating monuments and in advertising, where “the more dazzling and attractive a light, the more suitable it is.” This last use turned out to be the most popular. In 1912 Claude installed the first-ever neon advertising sign in a Parisian barbershop on the Boulevard Montmartre. A large rooftop sign for the Italian vermouth maker Cinzano soon followed, along with illumination for the entrance of the Paris Opéra. Making the most of his new invention, Claude formed another company, Claude Neon, to sell franchises for neon signage. Despite a high price tag—$100,000 plus royalties—dozens of franchises opened around the world, especially in major American cities. Neon was on its way to becoming a household name. Though the earliest neon signs were relatively simple—the range of colors and animation would come later—business owners competed with each other to trace their signatures on buildings and rooftops. Claude’s signage monopoly lasted through the 1920s, eventually crumbling as his patents expired and former employees leaked his trade secrets.

A New Sign Language
The first neon signs in the United States did not appear in New York or Las Vegas (which had a population of just a few thousand people in the early 1920s) but in the boomtown of Los Angeles. Entrepreneur Earle C. Anthony was a pioneer in several modern businesses, notably radio, automobiles, and gas stations. In 1915 he founded the first California dealership for the Packard Motor Car Company, a luxury brand, and remained the sole Packard distributor in the state through the 1950s. Anthony saw Claude’s neon signs on a visit to Paris and in 1923 commissioned a stylish promotion for his downtown showroom: two signs, each with “Packard” in elegant script, traced in orange neon tubing with a clear blue border (most likely produced by adding mercury to the neon). The signs cost $1,250—about half the price of a 1923 five-passenger Packard Single-Six Touring car—but Anthony’s investment paid off. The signs were a sensation, reportedly causing traffic jams as people stopped to marvel at their intense glow.
The first neon signs in the United States did not appear in New York or Las Vegas but in the boomtown of Los Angeles.
From that point on, neon was unstoppable. It truly was “the new one,” a symbol of modern industry, commerce, and progress in a world still recovering from the traumas of World War I and the effects of the Great Depression.
“In New York and London, in Denver and Shanghai, along the Main Streets of the world, dusk brings forth a million vivid electric signs that make the night alive. There is a new sign language . . . written in glass!” proclaimed a 1937 advertisement for Corning Glass Works, which supplied tubes for neon signs. Claude’s use of neon at the Paris Motor Show was perhaps prophetic since neon soon became an integral part of automobile culture, particularly in the United States. As the American interstate highway system developed, neon signs across the country promoted businesses that catered to motorists: gas stations, diners, motels, and roadside attractions. And New York, Los Angeles, and especially Las Vegas became famous for the countless neon signs that enticed people with visions of nighttime pleasures, both accepted and forbidden: going to the movies or the theater, dining in restaurants, dancing, drinking, gambling, and sex.
Many people learned how to make neon signs by working with established sign makers, but a few trade schools (notably the Egani Neon Glassblowing School in New York City) also taught the painstaking technique. Working from a design traced on an asbestos sheet, a sign maker heated a glass tube over a burner or in a torch to create bends and curves, blowing frequently through the hot tube to keep it from collapsing. Further steps included attaching electrodes to the tube, evacuating the air inside, and “bombarding” the interior with high voltage to clean the glass. After small amounts of gases were pumped in—usually a neon-argon mixture, sometimes with a little mercury—and the tube was sealed, it was “aged” with electrical current to remove impurities from the gas and ensure a steady luminosity. The completed tubes were then mounted on a metal supporting plate, which was often coated in enamel for durability and to enhance the light from the tubes. Once the electrical apparatus was added, the sign was complete.
Adjusting the gas mixture and tinting or coating the tubes allowed for more than 40 different color combinations. Even with the limitations imposed by this fragile and difficult medium, many forms and shapes were possible: block letters, flowing cursive script, combinations of lines and geometric designs, and pictures of all sorts, from the humble shoe or fish in a shop window to the elaborate, large-scale moving signs aptly called “spectaculars.” Animated by complex timing devices that turned tubes on and off in succession, these signs dazzled onlookers with outlines of speeding trains, gigantic dancing showgirls, or drinks poured into immense glasses. Spectaculars were masterpieces of art and technology, requiring hundreds of feet of tubing and miles of electrical wiring.
Counting on Neon
Neon didn’t just glow in signs. At the dawn of the computer age in the 1950s and 1960s neon tubes were key components of some digital circuits. This unusual application was possible because of the way electricity functions in neon tubes. Electrons flow through a tube only when it is lit. The voltage needed to light a neon tube is higher than that needed to keep it on. By maintaining a tube at a voltage somewhere between on and off, small increases or decreases in voltage could be used to control the current (seen as light). Precise regulation of flow of current allowed the tube to be used as a binary switch to control digital circuits.
These neon switches could be linked up to create circuits needed for a range of applications, from the simple arithmetic of accounting to the measurement of events that occur faster than humans can count, such as in radioactive decay. “Glow lamps filled with Airco neon help computers think faster,” a 1961 magazine advertisement explained. “Without the neon glow lamp the dazzling speed, compactness and economy of the electronic computer would not be possible. . . and many of the spectacular advances that are now being made in business and defense technologies would be slowed down considerably.”
Neon truly was ‘the new one,’ a symbol of modern industry, commerce, and progress in a world still recovering from the traumas of World War I and the effects of the Great Depression.
Neon tubes had another advantage for computers and technical equipment. Since they ran cooler and more efficiently than incandescent bulbs, they could be used as indicator lights and displays. A common type of neon display was the Nixie tube (short for “Numeric Indicator eXperimental No. 1”), introduced in 1955. This small neon bulb had wires shaped like numerals, letters, or other symbols, one in front of the other, that lit up when the current was turned on.
Neon’s biggest electronic triumph may have been the world’s first electronic desktop calculators, the ANITA Mk VII and Mk VIII, invented by Norbert Kitz of the Bell Punch Company in England. The ANITA machines, whose name was an acronym for A New Inspiration To Arithmetic (or Accounting), had a Nixie tube–like display. An advertisement highlighted this feature: “Answers are recorded in large lit-up figures which defy you to misread them.”
Inside the ANITAs, neon-filled switching tubes drove the calculating logic. Introduced in 1961–62 at a cost of 355 pounds sterling (about $1,000 at the time, or $7,500 today), ANITAs sold at the rate of 10,000 per year to such businesses as banks, accounting firms, and department stores. With a footprint of about 1-by-1.5 feet (31 centimeters by 46 centimeters) and a weight of over 30 pounds (14 kilograms) each, ANITA calculators were large by modern standards. They were, however, quieter and faster than earlier mechanical calculators, and much smaller and cheaper than the huge (and hugely expensive) computers of the day. As a 1965 article explained:
But by the 1970s neon tubes in computers were largely obsolete. Transistors became the preferred electronic switching elements, and light-emitting diodes (LEDs) began to replace Nixie tubes in displays. Neon, however, still glows brightly in do-it-yourself electronics. Today, avid hobbyists seek out vintage Nixie tubes for hand-built clocks and the occasional eye-catching wristwatch—and some have even created retro-style neon circuits.
Neon Old and New
Neon’s supreme reign in signage was also relatively brief. Nighttime blackouts during World War II darkened neon signs around the United States, and many large ones were never relit. Cheaper, low-maintenance signs made possible by new kinds of plastics and fluorescent tubes replaced them. Defunct neon signs, considered eyesores in many municipalities, were scrapped, though dedicated collectors and preservationists fought to preserve and restore these pieces of genuine Americana.
Nowadays, most neon signs are small and simple, such as “Open” signs for stores or beer advertisements for bars. The late 1970s, however, saw the beginnings of a small-scale neon revival that is still ongoing. Attracted by neon’s unique look and retro appeal, sign makers bent tubes for signs in old and new styles, passing on their skills to a new generation; architects used neon to accent buildings inside and out; and artists pushed the medium in new directions, drawing with light to create unique abstract sculptures.
Even if giant television screens and lighted billboards have replaced the extravagant neon “spectaculars” of old in New York City’s Times Square and elsewhere, neon still illuminates the night in cities and towns worldwide, from Las Vegas to Tokyo and beyond. And what of the first neon signs in the United States? Packard cars are long gone, but Earle C. Anthony’s showroom remains standing in downtown Los Angeles. Over the entrance a replica neon sign advertises the building’s new function in a brilliant blue-white glow: “Packard Lofts.”
Xenon Freezes the Moment
All the noble gases produce striking illumination, but xenon played an important role in the development of high-speed or flash photography. Improving on the chemical flashes and electric sparks of photography’s early days, xenon-filled tubes produce brief, intense bursts of light that can freeze motion on film.
Harold Edgerton, a professor of electrical engineering at the Massachusetts Institute of Technology, originated high-speed photography using many kinds of flash technology, including strobe lamps. One of his most iconic images shows a crown-shaped milk droplet captured in mid-splash. His first flash lamps used mercury or argon, but Edgerton switched to xenon in the 1940s. Xenon flash tubes were an excellent choice, particularly for color photography. Unlike neon’s blazing red or argon’s purple, xenon’s glow in a discharge tube is similar to sunlight, enabling photographers to better capture natural color indoors.
The original xenon flash tubes and associated electronics developed in the 1940s were too cumbersome for anyone but professional photographers to use. By the 1970s, however, miniaturized xenon flash tubes were small enough to become standard features of regular cameras. While light-emitting diodes (LEDs) are now often used as flashes in cell-phone cameras and some digital cameras, many cameras, particularly high-end ones, still take advantage of the intensity and natural color of xenon light. So next time someone takes your picture with a flash, think of xenon and smile.




