Refrigeration by Evaporation
How heat can make it cooler.
How heat can make it cooler.
At the Science History Institute, we have a wide range of collection objects that tell the stories of the foods we eat. Food science innovations have benefited the public through the supply of safe products that are nutritious, abundant, and accessible. One such innovation is Louis Pasteur’s pasteurization process, which was invented and patented in 1865 and continues to be used to assist in the preservation of foods and beverages.


Preservation is central to the safety of the foods we eat. When properly utilized, it reduces the risk of contamination by pathogenic bacteria, viruses, or parasites. Drying, smoking, fermenting, and canning were all preservation techniques commonly used in the U.S. prior to the introduction of commercially produced ice in the 19th century. The widespread use of cold storage preservation shifted the average U.S. diet to include more fresh meat, dairy products, fruits, and vegetables. In the 20th century, mechanical refrigeration rapidly displaced the use of ice in cold food storage, and today you would be hard-pressed to find a home in the U.S. without a fridge.
Contemporary domestic and commercial refrigerators utilize a vapor-compression refrigeration system first patented by Jacob Perkins in 1834. To produce cool temperatures, a liquid chemical compound—a refrigerant—is rapidly vaporized through compression. These refrigerants evaporate at an extremely low temperature and as they circulate, heat is absorbed and removed from the space to be cooled. While highly effective, these refrigerants have a long history of being extremely volatile and highly toxic.

The evaporative cooling process through which our electric refrigerators create artificial cool air is more humbly demonstrated by a seemingly unsophisticated object within our collection: the “Polar” Milk Cooler. The milk cooler’s application is simple: the terracotta vessel is soaked in water and then placed over a milk bottle. The water molecules evaporate from the surface of the porous earthenware, creating an interior cool enough to delay spoilage of the milk.
Fun fact: The first glass milk bottle was created to further ensure the milk’s safety. The “Common Sense Milk Bottle” was invented by druggist Dr. Hervey Thatcher in 1885 to provide sanitary milk delivery. Prior to the use of glass bottles, dairy wagons transported milk in bulk using large metal containers. The milk was then ladled into residents’ own personal containers at each stop. Our “Polar” Milk Cooler was manufactured sometime in the 1930s, but the evaporative cooling process has been ingeniously employed by humans since the 2nd and 3rd millenniums BCE.
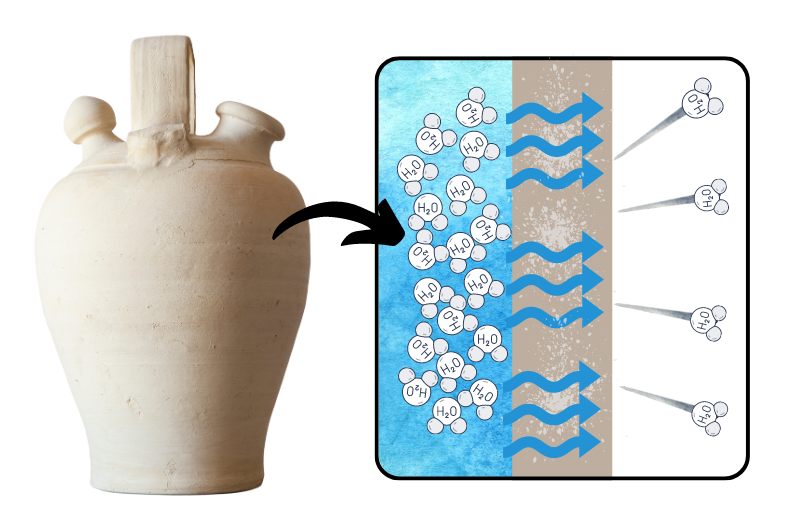
The Spanish botijo is a traditional vessel made of unglazed terracotta that supplies cool drinking water through “sweating.” Archeological evidence of their use was found at the Puntarrón Chico de Beniaján archeological site, which holds remnants of Argaric settlements from the early Bronze Age. As water slowly diffuses through the porous clay walls, it evaporates from the surface of the pitcher. Through this evaporation, the temperature of the water within the vessel can be reduced by as much as 10 degrees Celsius (or 18 degrees Fahrenheit). Similar earthenware sweating vessels are also used in India and Pakistan. With certainty we can presume the “Polar” Milk Cooler was modeled from these traditional pitchers and pots.
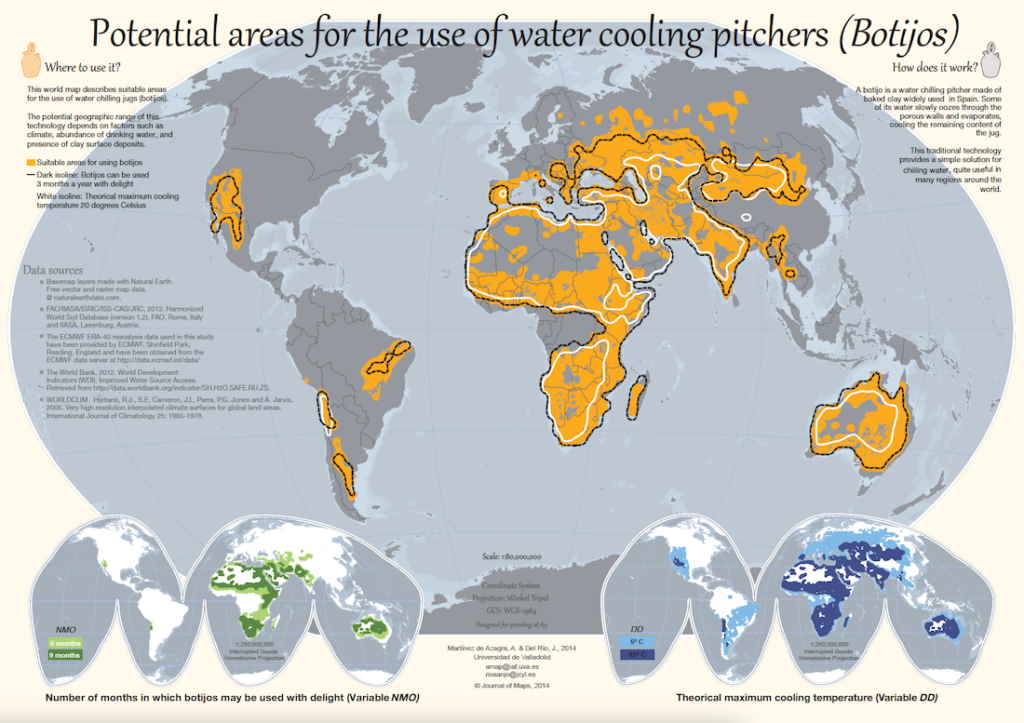
One important requirement for the successful use of evaporative cooling is the presence of dry heat. A humid environment, like Philadelphia, renders the process ineffective as the concentration of water vapor in the air is too high (sorry Philly!). But there are portions of the Southwest U.S. that are dry and hot enough to allow its inhabitants to enjoy refreshing water from a botijo. Researchers Andrés Martínez de Azagra and Jorge Del Río published a paper in 2014 that presented a world map of potential areas that could make use of water-cooling pitchers. The authors found that areas with high temperatures and low humidity as well as access to potable water and clay surface deposits span large portions of Asia, Europe, Australia, and Africa, and smaller regions in North and South America.
Evaporative cooling has also been engineered into the traditional architecture of Iran to provide cold food storage and comfort in an otherwise uncompromising climate. Dating back to 400 BCE, Yakhchāls (or ice pits) were used to harvest, store, and preserve ice generated during the winter months. The structure of the Yakhchāl comprises a large shading wall, a shallow pool, and an ice reservoir. The shallow pool would be filled with water and during the colder winter nights, this water would freeze. This ice was harvested during the day and stored in the reservoir with the shading wall providing protection from the sun’s radiation.
The reservoirs to store the ice were often built below ground to ensure temperature control and insulation. Ice collected was used to preserve meat, dairy products, and fresh fruits and vegetables. This provided access to an abundance of safe food during dangerously hot summer months, a feat accomplished by only using the energy of the sun and the wind. In a 1978 Scientific American article, mechanical engineering professor Mehdi N. Bahadori wrote, “A passive cooling system exploits the very features of the climate it seeks to overcome . . . [It] demonstrate[s] the possibilities of working with rather than against the external environment.

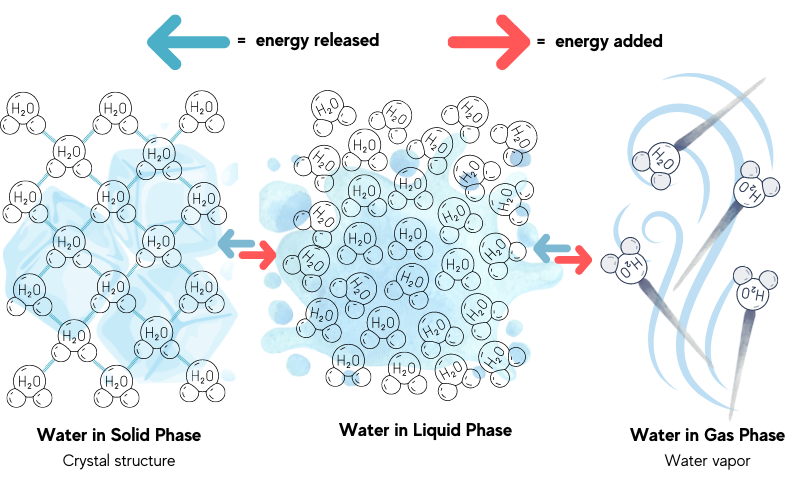
So, how can the energy of the sun and the wind be cooling, in a desert no less? The process through which evaporation cools is the transfer of heat that occurs during a phase change—a substance shifting from one physical form to another. Freezing, melting, and evaporation of water are all examples of a phase change. These phase changes will either release energy or use energy. How much energy is released or used depends on the strength of intermolecular forces, or the attraction between whole molecules.
Water is a substance that exhibits a strong attraction between molecules, called hydrogen bonding. A great deal of energy is required to break hydrogen bonds to allow water molecules to move farther apart. The energy needed to evaporate liquid water on the surface of the botijo and milk cooler comes from its surroundings: the hot and arid air. The reverse occurs within the shallow pools of the Yakhchals: the water in the pool loses heat to the air during a cold desert night. Enough heat energy is released from the liquid water to initiate a phase change and ice is formed.
We ourselves are intimately familiar with evaporative cooling, as our body’s physiology brilliantly exploits this process through perspiration. When our internal body temperature begins to rise on a hot summer day, eccrine glands secrete sweat and water accumulates on the skin. The heat from the air energizes these water molecules and a portion of them vaporize (evaporate). The water molecules left on the skin now have a lower amount of energy. Heat (thermal energy) then transfers from your skin to the water molecules with lower energy. The transfer of energy from your skin to your sweat gives you the sensation of feeling cooler.
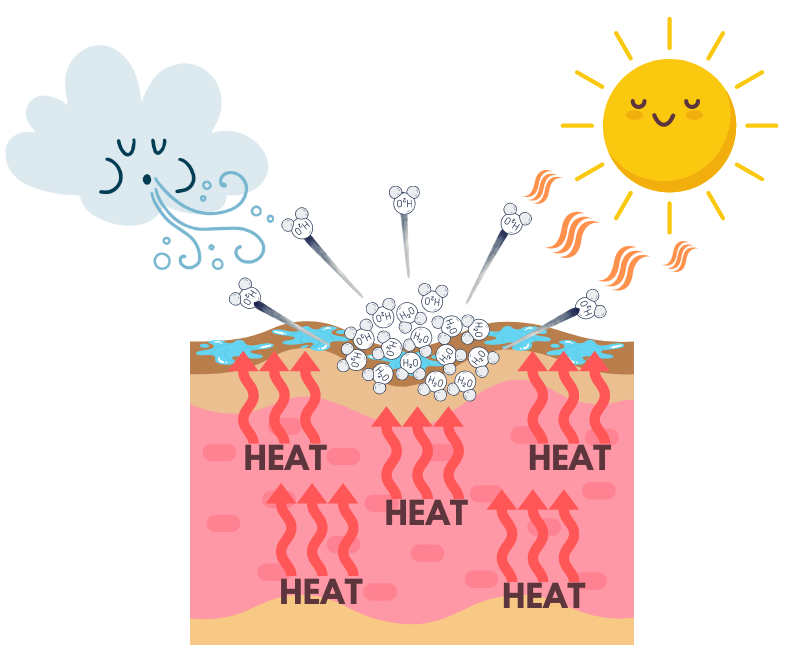
Sweating is literally and figuratively cool, and a thermoregulating mechanism unique to just a handful of mammals. In fact, pigs—who are often referred to when one is saturated in stinky sweat—have only a limited number of functioning sweat glands. They use behavioral thermoregulation, such as wallowing in water or mud, to assist in lowering their body temperature. So the next time you find yourself overly perspiring on a sweltering summer day, I encourage you to exclaim, “Woowee! I’m sweating like a “Polar” Milk Cooler on a hot dry day!”
Featured image: Illustration of “The Hydrologic Cycle” from the USDA’s Water: The Yearbook of Agriculture 1955.
If you enjoyed sinking your teeth into the history and science of evaporative cooling, you will undoubtedly appreciate our new Lunchtime: The History of Science on the School Food Tray exhibition. Lunchtime offers a novel historical perspective on the efforts made to feed children in U.S. schools and will be on view in our museum until January 2026. We look forward to seeing you there!
Memory, materials, and the history of science in the Eugene Garfield Papers.
Explore the history of science behind U.S. efforts to feed schoolchildren with Lunchtime exhibition curator Jesse Smith.
Unwrapping the mystery of a Styrofoam Santa in our collections.
Copy the above HTML to republish this content. We have formatted the material to follow our guidelines, which include our credit requirements. Please review our full list of guidelines for more information. By republishing this content, you agree to our republication requirements.