A Chilling History
On the science and technology of portable coolers.

On the science and technology of portable coolers.
With the dog days of summer upon us here in Philadelphia, many people find relief from sweltering city temperatures by jumping in a pool, visiting the beach, or camping in the mountains. One invaluable item that has become synonymous with outdoor leisure and recreation is the portable cooler or ice chest.
While today the portable cooler may seem like an ancient tool compared to the swanky electric refrigerators in our homes, ice was a dominant form of food preservation in the United States for centuries. Icehouses were structures designed to preserve ice year-round. They ranged in size from small domestic outbuildings to large commercial warehouses for harvested ice.
In the 18th century, private icehouses for domestic use were a luxury enjoyed by wealthy landowners; others were built by tavern and restaurant owners. Prior to the 1800s, the vast majority of Americans preserved food through drying, smoking, salting, canning, and storing in cold cellars, springhouses, and dairies. As ice harvesting tools, technology, and transport improved in the 19th century, commercially produced ice became more available. Consequently, beginning in the 1830s, the average U.S. diet shifted to include more fresh meats and vegetables, in addition to iced drinks, desserts, and ice cream.

The first domestic “refrigerator” invented and patented in the U.S. looked more like the portable coolers we know today. Thomas Moore invented his “Refrigiratory” for the transport of butter to markets, for which he received a patent in 1803. It was a cedar oval tub with a hinged lid and insulated on the outside with rabbit fur and coarse woolen cloth. Its tin inner chamber held 22 one-pound pieces of butter and was cooled by ice placed between the interior tin and exterior cedar. Fellow inventor Thomas Jefferson owned one of Moore’s refrigerators and used it for many years. Jefferson’s granddaughter, Ellen, lamented on its use in a letter she wrote to her mother in 1819 during a trip to Jefferson’s Poplar Forest in 1819:
Grand papa insisted on our using that filthy cooler, (refrigerator, I believe he calls it,) which wasted our small stock of ice, and gave us butter that run about the plate so that we could scarcely catch it, and wine above blood-heat . . .
We feel you, Ellen.
By the end of the 1800s, cold was the prevailing method for preserving food, and private homes began storing perishable food in cork insulated “iceboxes” usually made of wood and lined with tin or zinc, as these metals resist corrosion from water. A large block of ice stored inside chilled the air. Introduced in 1927, General Electric’s Monitor Top was the first mass produced electric refrigerator to become widely popular in private homes. In 1935 New Deal loans encouraging Americans to switch to electric led to a huge increase in electric refrigerator sales, and electric refrigeration ownership grew further with the economic prosperity some experienced after World War II.
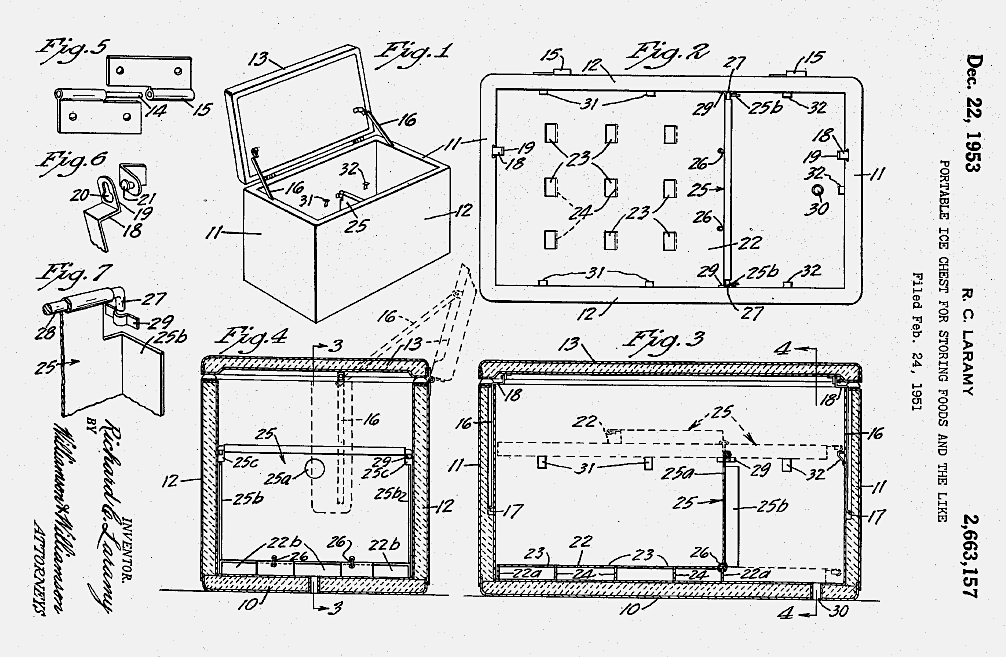
By 1957, 90% of U.S. homes had electric refrigerators, compared to fewer than 10% in the United Kingdom. Some consumers also began celebrating their newfound abundance and leisure-time through casual outdoor dining. Postwar suburban families invested in grills and other accessories for their backyards and other recreational outings. In 1953 Richard C. Laramy received a U.S. patent for his invention of “a portable ice chest for storing and refrigerating foods and the like.” Portable coolers soon became a staple item for camping, trips to the beach, and picnics.
How do portable coolers work? Within icehouses, iceboxes, and portable coolers, cooling happens when water changes from its solid state into a liquid. Energy is required to convert ice to liquid, but where does the energy come from? Because energy cannot be created or destroyed, surroundings play an important role in energy changes. Energy may be absorbed from the surroundings to provide thermal energy needed to melt ice. Additionally, the surroundings may receive the thermal energy released when water freezes.
Within a portable cooler, the surroundings are defined as everything other the water molecules. Since this phase change is taking place in a cooler filled with items we want to keep cool, even the cooler itself and the food and drink inside are considered the surroundings. So, too, is the air, the table, or ground the cooler is sitting on, your hand reaching in and out of the cooler, and even the rest of the outside world. The water molecules (ice) are the system.
This is the energy transfer that happens inside a portable cooler: When the warmer, faster-moving molecules of the surroundings collide with the colder, slower-moving ice molecules, part of the kinetic energy of the surroundings’ molecules are used to move the solid water molecules out of their crystal lattice structure (this is melting).
The total amount of energy transferred from the surroundings to the water molecules in the ice cubes is called heat. These collisions will continue to transfer energy until the temperatures of the ice molecules and molecules in the surroundings are identical. This is called thermal equilibrium, and when this equilibrium has been reached, the average kinetic energy of both types of particles is equal. If thermal equilibrium is maintained there is no change in the average kinetic energy of either the system or surroundings. Collisions between molecules will still occur, but the number of collisions resulting in an energy gain versus those collisions resulting in an energy loss are equal.
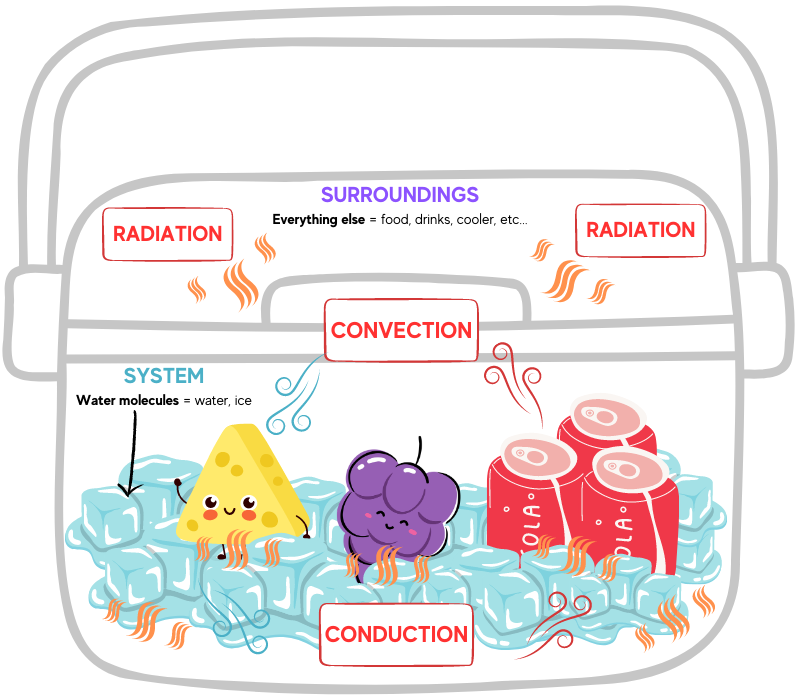
Thermal energy is transferred via convection, conduction, and radiation. Convection transfers energy from one place to another through the movement of heated fluids that carry thermal energy away from the source, such as atmospheric air that makes us sweat or boiling water that cooks an egg. Conduction is the movement of thermal energy from one solid to another; think of the pain of walking barefoot on a hot beach (yowch!).
Radiation energy transfer does not rely on any contact between the heat source and the heated object, such as sunlight that warms atmospheric air. Thermal energy transfer occurs both inside and outside the portable cooler, and a cooler’s materials insulate its contents from radiation, convection, and conduction. Tin, zinc, various woods, cork, thermoplastics, and expanded polystyrene foam have specific physical properties—such as nonconductivity and water resistance—that help achieve thermal equilibrium.
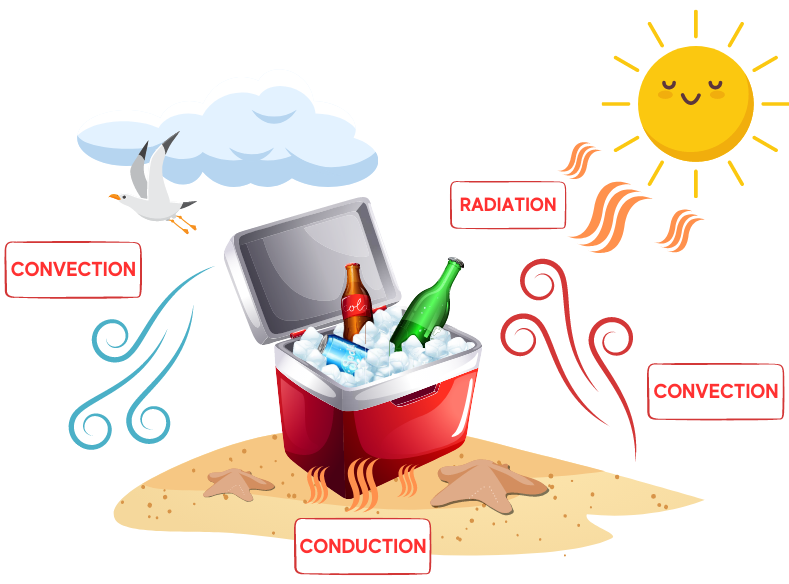
Since the use of plastics exploded in the 1950s, most portable coolers today are made entirely from synthetic materials such as polypropylene and expanded polystyrene (EPS) foam, which are light and inexpensive but still provide the insulation needed to reduce heat transfer. There is a common misconception that food packaging items, such as portable coolers, are made from Styrofoam brand foam. Styrofoam is the trademark brand of closed-cell, extruded polystyrene foam patented by Dow Chemical in 1944, and used mainly for building insulation and buoyancy devices.

EPS products are made through an expansion process in which small beads of resin are warmed and then squeezed together into the desired shape. Expandable polystyrene beads were introduced in 1954 by Koppers Company, Inc. with their Dylite products. (Impress your friends with this pedantic fact at your next cookout or picnic!)
Who knew there could be so much history and science encapsulated in one humdrum recreation accessory?
If you would you like to learn more about summer-themed science, join us for First Friday: Science “Down the Shore!” on Friday, August 4, 5pm to 7pm. You can meet me and my fellow gallery guides who bring our museum’s historical artifacts to life through themed tours, object interpretation, and hands-on learning.
Featured image: National Technical Laboratories Company Picnics photograph, 1946. Science History Institute.
Mapping Philadelphia’s industrial past with digital tools.
Memory, materials, and the history of science in the Eugene Garfield Papers.
Explore the history of science behind U.S. efforts to feed schoolchildren with Lunchtime exhibition curator Jesse Smith.
Copy the above HTML to republish this content. We have formatted the material to follow our guidelines, which include our credit requirements. Please review our full list of guidelines for more information. By republishing this content, you agree to our republication requirements.